Abstract
Severe acute pancreatitis (SAP) is associated with systemic complications and high mortality rate in dogs. Mesenchymal stem cells (MSCs) have been investigated for their therapeutic potential in several inflammation models. In the present study, the effects of canine adipose tissue-derived (cAT)-MSCs in a rat model of SAP induced by retrograde injection of 3% sodium taurocholate solution into the pancreatic duct were investigated. cAT-MSCs labeled with dioctadecyl-3,3,3′-tetramethylindo-carbocyanine perchlorate (1 × 107 cells/kg) were systemically administered to rats and pancreatic tissue was collected three days later for histopathological, quantitative real-time polymerase chain reaction, and immunocytochemical analyses. Greater numbers of infused cAT-MSCs were detected in the pancreas of SAP relative to sham-operated rats. cAT-MSC infusion reduced pancreatic edema, inflammatory cell infiltration, and acinar cell necrosis, and decreased pancreatic expression of the pro-inflammatory cytokines tumor necrosis factor-α, interleukin (IL)-1β, -6, -12, -17, and -23 and interferon-γ, while stimulating expression of the anti-inflammatory cytokines IL-4 and IL-10 in SAP rats. Moreover, cAT-MSCs decreased the number of clusters of differentiation 3-positive T cells and increased that of forkhead box P3-positive T cells in the injured pancreas. These results indicate that cAT-MSCs can be effective as a cell-based therapeutic strategy for treatment of SAP in dogs.
Acute pancreatitis is a common disease in dogs. Although most cases are self-limiting and fully reversible, some progress to severe acute pancreatitis (SAP), which leads to systemic complications such as multi-organ failure and diffuse intravascular coagulation [827]. Mortality rates among dogs with SAP are 27% to 42% [813]. To date, no effective treatment strategies have been developed, indicating the need for a better understanding of the pathophysiology of SAP. A breed predisposition has been reported for acute pancreatitis that deteriorates into SAP, implying that the disease is related to hereditary mutations [27], including those that cause auto-activation of trypsin, resulting in pancreatic edema, death of acinar cells [17], and an inflammatory response mediated by cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, -6, -12, -4, and -10, interferon (IFN)-γ released by macrophages and T cells [3134]. Overproduction of these inflammatory cytokines can lead to systemic manifestations, multi-organ failure, or death [30].
Mesenchymal stem cells (MSCs) have recently been investigated for their therapeutic potential in the treatment of SAP. Previous studies have shown that MSCs regulate immune responses in inflammatory bowel disease, sepsis, encephalomyelitis, and arthritis models [1111239]. In addition, human MSCs have been reported to mitigate SAP by suppressing inflammation in a rodent model [192028]. Although canine MSCs can differentiate into multilineage cells [2632], few studies have focused on their immunomodulatory effects. Therefore, the present study investigated the therapeutic effects of canine adipose tissue-derived cAT-MSCs in a rat model of SAP, as well as their modulation of host immune response.
Canine adipose tissue was obtained from a healthy dog < 1 year old during routine spaying at Seoul National University Veterinary Medicine Teaching Hospital (SNU VMTH), and MSCs were isolated as previously described [36]. Briefly, the tissue was washed three times with phosphate-buffered saline (PBS; PAN-Biotech, Germany) containing 100 U/mL penicillin and 100 g/mL streptomycin, then cut into small pieces and digested for 1 h at 37℃ with collagenase type IA (1 mg/mL; Sigma-Aldrich, USA). The enzymatic activity was inhibited with Dulbecco's Modified Eagle's Medium (DMEM; PAN-Biotech) containing 10% fetal bovine serum (FBS; PAN-Biotech). Following centrifugation at 1,200 × g for 5 min, the pellet was filtered through a 70 µm Falcon cell strainer (Fisher Scientific, USA) to remove debris, then incubated in DMEM containing 10% FBS at 37℃ in a humidified atmosphere of 5% CO2. After 48 h, cultures were washed with PBS to remove non-adherent cells and incubated with fresh medium, which was changed every 48 h until cells reached 70% to 80% confluence. The cells were then repeatedly subcultured under standard conditions. Before their use in experiments, cells were characterized for the expression of several stem cell markers by flow cytometry using fluorescein isothiocyante (FITC)-, phycoerythrin (PE)-, or allophycocyanin (APC)-conjugated antibodies against the following proteins: cluster of differentiation (CD)29-FITC, CD31-FITC, CD34-PE, and CD73-PE (BD Biosciences, USA), and CD44-FITC, CD45-FITC, and CD90-APC (eBiosciences, USA). Cells were analyzed using a FACSAria II system (BD Biosciences). Cellular differentiation was evaluated using the kits (Gibco, USA) of the StemPro Adipogenesis Differentiation, StemPro Osteogenesis Differentiation, and StemPro Chondrogenesis Differentiationaccording to the manufacturer's instructions, followed by Oil Red O staining, Alizarin red staining, and Alcian blue staining, respectively.
Male Sprague-Dawley rats (Nara Biotech, Korea) weighing 190 to 220 g were used for experiments. All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee of SNU (protocol No. SNU-150820-1). Rats were randomly divided into the following four groups: SHAM+PBS (n = 4), SHAM+MSC (n = 4), SAP+PBS (n = 8), and SAP+MSC (n = 8). SAP was induced by retrograde injection of sodium taurocholate (TCA; Sigma-Aldrich) into the pancreatic duct as previously described [16]. Briefly, rats were anesthetized and laparotomized at the midline. After exposing the first loop of the duodenum and the pancreas, the proximal common bile duct was clipped with a microclamp. A needle catheter was then inserted through the middle common bile duct via the duodenal papilla and ligated, after which a solution of 3% TCA in saline (1 mL/kg) was injected into the common bile-pancreatic duct over a 60 sec period. The microclamp and ligature were subsequently removed and the abdomen closed. Rats in SHAM groups were also anesthetized and laparotomized at the midline, but their abdomens were closed without additional manipulations. Following surgery, cAT-MSCs labeled with chloromethylbenzamido-1,1′-dioctadecyl-3,3,3′-tetramethyli ndo-carbocyanine perchlorate (CM-DiI; Invitrogen, USA) were administered via the tail vein to rats in the MSC groups (1 × 107 cells/kg in 200 µL PBS), while an identical volume of PBS was administered via the same route to PBS groups. Rats were sacrificed 3 days after these procedures. Blood samples were centrifuged to obtain serum, which was stored at −80℃ until use. Pancreatic tissue was collected and washed in cold PBS. After weighing the pancreas, half of the tissue was fixed in 10% formaldehyde and embedded in paraffin, while the other half was stored at −80℃ until use.
Paraffin-embedded tissue samples were cut into 4 µm sections that were stained with hematoxylin and eosin and examined under a light microscope. A total of 20 randomly selected fields per group were scored in a blinded manner. Pancreatic acinar cell injury was scored on a scale from 0 to 4 based on the degree of edema (0, absent; 1, expanded interlobar septa; 2, expanded interlobular septa; 3, expanded interacinar septa; 4, expanded intercellular septa), infiltration (0, 0–1 white blood cells [WBCs]/high-power field [HPF]; 1, 2–10 WBCs/HPF; 2, 11–20 WBCs/HPF; 3, 21–30 WBCs/HPF; 4, > 30 WBCs/HPF), and necrosis (0, absent; 1, 1–4 necrotic cells/HPF; 2, 5–10 necrotic cells/HPF; 3, 11–15 necrotic cells/HPF; 4, > 15 necrotic cells/HPF).
Serum amylase and lipase activities were measured using the EnzyChrom α-Amylase Assay and QuantiChrom Lipase Assay kits (both from BioAssay Systems, USA) according to the manufacturer's instructions. Serum IFN-γ and IL-10 levels were measured using rat-specific Quantikine enzyme-linked immunosorbent assay kits (R&D Systems, USA) according to the manufacturer's instructions.
Approximately 100 mg of pancreas tissue were homogenized and RNA was extracted using the Easy-BLUE Total RNA Extraction kit (iNtRON Biotechnology, Korea). Next, cDNA was synthesized using LaboPass M-MuLV Reverse Transcriptase (Cosmogenetech, Korea) according to the manufacturer's instructions. Samples were assayed in duplicate in 10 µL AMPIGENE qPCR Green Mix Hi-ROX with SYBR Green dye (Enzo Life Sciences, USA) using 1 µL cDNA and 400 nM forward primer and reverse primers (Cosmogenetech). The cycling conditions were 95℃ for 2 min, followed by 40 cycles of 95℃ for 5 s and 60℃ for 25 sec. Expression levels were normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
The blood of a healthy canine donor was obtained from SNU VMTH and diluted with an equivalent volume of PBS, then layered over Ficoll-Paque PLUS (GE Healthcare Life Sciences, Sweden) in conical tubes. Following centrifugation at 400 × g for 30 min, the cell layer was collected and washed twice with PBS. cPBMCs were then resuspended in Roswell Park Memorial Institute (RPMI)-1640 medium (PAN-Biotech) containing 10% FBS.
Splenocytes were isolated from rats as previously described [3]. Briefly, rat spleens were removed and cut into small pieces in a cell strainer with PBS. The tissue was then crushed using the plunger from a 1 mL syringe. Next, the homogenized cell suspension was transferred to a tube and the cell pellet was obtained by centrifugation and resuspended in Red Blood Cell Lysis Buffer (Sigma-Aldrich). After two washes with PBS, splenocytes were resuspended in RPMI-1640 containing 10% FBS.
Isolated canine PBMCs and rat splenocytes were used in the MLR. Briefly, cAT-MSCs were treated with 25 µg/mL mitomycin C (Sigma-Aldrich) for 1 h at 37℃. After five washes, cAT-MSCs were seeded in a 96-well plate at 1 × 104, 1 × 103, and 1 × 102 per well to determine the cAT-MSC-to-cPBMC ratio dependency. After 6 h, mitomycin C-treated cAT-MSCs were attached, and cPBMCs stimulated with 2 µg/mL concanavalin A (ConA; Sigma-Aldrich) were added to each well of cAT-MSCs cultured at 1 × 105/well. After 5 days, cPBMC proliferation was assessed with the BrdU Cell Proliferation Assay Kit (BioVision, USA) according to the manufacturer's instructions. For MLR with conditioned medium (CM), cAT-MSCs were seeded in 6-well plates at 3 × 105/well, and the medium was changed after 24 h. After 5 days, the CM was harvested and centrifuged to remove debris. The MLR was carried out in CM as described above. The procedure was then repeated using rat splenocytes.
Paraffin sections were cut at a thickness of 4 µm for immunolabeling. Sections were deparaffinized and rehydrated, and antigen retrieval was carried out in 10 mM citrate buffer. Sections were then washed and blocked with blocking buffer containing 5% normal goat serum (Gibco) and 0.3% Triton X-100 (Sigma-Aldrich) for 1 h, then incubated overnight at 4℃ with mouse monoclonal anti-CD3 (1 : 100) and anti-forkhead box (Fox)P3 (1 : 100) antibodies (both from Santa Cruz Biotechnology, USA). After three washes, the sections were incubated with secondary antibody (1 : 200; Santa Cruz Biotechnology) for 1 h at room temperature in the dark, then washed three times and mounted in Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (Vector Laboratories, USA). Finally, the samples were visualized with an epifluorescence microscope. Immunoreactive cells were counted in 20 random fields per group.
Cells isolated from canine adipose tissue were identified by immunophenotyping and multilineage differentiation. The cells had a high expression of known MSC markers such as CD29, CD44, CD73, and CD90, and did not express CD31, CD34, or CD45 (panel A in Fig. 1). The cAT-MSCs had multilineage plasticity, as demonstrated by their potential for adipogenic, osteogenic, and chondrogenic differentiation (panel B in Fig. 1).
Pancreatic tissue samples from SAP groups exhibited edema, inflammatory cell infiltration, and necrosis of acinar cells compared to those from SHAM animals. The severity of pancreatic injury was markedly diminished in the SAP+MSC relative to the SAP+PBS group (p < 0.001; panel A in Fig. 2). After cAT-MSC infusion, serum amylase and lipase activities, which are important markers of pancreatic function, decreased by 45% (panel B in Fig. 2) and 32% (panel C in Fig. 2), respectively, relative to the SAP+PBS group. In addition, the pancreas-to-body weight ratio, a measure of pancreatic edema, was reduced in the SAP+MSC relative to the SAP+PBS group (p < 0.05; panel D in Fig. 2).
The capacity of infused cAT-MSCs to migrate to the injured pancreas was evaluated by fluorescence microscopy and reverse transcription PCR analysis. CM-DiI-labeled cAT-MSCs were identified by their red fluorescence. There was a greater number of CM-DiI-labeled cAT-MSCs in the pancreatic tissue of the SAP+MSC than the SHAM+MSC group, whereas no fluorescent cells were detected in the PBS groups (panel A in Fig. 3). Consistent with this observation, the mRNA level of canine ribosomal protein S5 (cRPS5) was higher in the SAP+MSC than in the SHAM+PBS group (panel B in Fig. 3), confirming the presence of cAT-MSCs.
The mRNA expression of pro- and anti-inflammatory cytokines in pancreatic tissue was evaluated by quantitative real-time PCR. The levels of TNF-α, IL-1β, -6, -12, -17, and -23 and IFN-γ were markedly increased after SAP induction. cAT-MSC infusion resulted in downregulation of these pro-inflammatory cytokines (p < 0.05 or p < 0.01; panel A in Fig. 4). Conversely, the expression of the anti-inflammatory cytokines IL-4 and -10 decreased by SAP induction, but increased in the SAP+MSC group relative to the SAP+PBS group (p < 0.05; panel A in Fig. 4). Furthermore, serum levels of IFN-γ and IL-10 were decreased by 30% and increased by 21%, respectively, in the SAP+MSC relative to the SAP+PBS group (panel B in Fig. 4).
We next evaluated the effects of cAT-MSCs on T cells, which release inflammatory cytokines. cAT-MSCs suppressed the proliferation of cPBMCs treated with ConA in a cAT-MSC:cPBMC ratio-dependent manner. At ratios of 1 : 10 and 1 : 100, cPBMCs proliferation was markedly suppressed (p < 0.001 and p < 0.05, respectively) relative to cells grown without cAT-MSCs. However, this was not observed at a ratio of 1 : 1,000. To assess the effects of soluble factors from cAT-MSCs on T cells, CM from cAT-MSC cultures was used as described above. The proliferation of cPBMCs stimulated with ConA in CM was suppressed (p < 0.05) relative to cells treated with control medium (panel A in Fig. 5). cAT-MSCs also inhibited the proliferation of rat splenocytes stimulated with ConA in a cAT-MSCs:rat splenocyte ratio-dependent manner. This effect was apparent at a 1 : 10 ratio and in CM (p < 0.001 and p < 0.05, respectively), but was not observed at ratios of 1 : 100 and 1 : 1,000 relative to splenocytes grown without cAT-MSCs or in control medium (panel B in Fig. 5). In addition, quantitative analysis of T cells detected in pancreatic tissue by immunocytochemistry revealed that the percentage of CD3+ T cells was decreased (p < 0.05; panel C in Fig. 5), whereas that of FoxP3+ regulatory T cells was increased (p < 0.01; panel D in Fig. 5) in the SAP+MSC relative to the SAP+PBS group.
Recent studies suggest that the anti-inflammatory function of MSCs can be applied to the treatment of SAP [16192028]. Most of these studies used MSCs isolated from human umbilical cord or bone marrow in animal SAP models; however, the therapeutic effects of canine MSCs have never been evaluated in an SAP model. Adipose tissue-derived MSCs have the advantage of being easy to isolate and available in larger quantities than other types of MSCs.
Intravenous administration of cAT-MSCs significantly mitigated SAP. Not only were serum amylase and lipase activities reduced, but histopathological manifestations including pancreatic edema, inflammatory cell infiltration, and acinar cell necrosis were improved. In addition, infused CM-DiI-labeled cAT-MSCs were detected more frequently in injured than in normal pancreas, indicating that they had migrated to the injured organ where they decreased and increased the levels of pro- and anti-inflammatory cytokines, respectively. The finding that the percentages of CD3+ T cells and FoxP3+ T cells were reduced and increased, respectively, in damaged pancreatic tissue after cAT-MSC administration suggests that the migrated cAT-MSCs suppressed inflammation by blocking T cell infiltration and inducing the proliferation of FoxP3+ regulatory T cells.
It is essential to determine if infused cells reach the sites of damage in MSC-based therapies. Previous studies have shown that injected MSCs can migrate to injured lungs, livers, kidneys, and colons [1115182934]. In the present study, the use of CM-DiI facilitated tracking of injected MSCs [2338]. A large number of systemically administered CM-DiI-labeled cAT-MSCs were detected in the injured pancreas, which was confirmed by PCR amplification of cRPS5, the canine-specific reference gene [5]. MSCs have the capacity to migrate to the site of inflammation in response to diverse cytokines and chemokines [721]; indeed, functional CC chemokine receptor type (CCR)1, CCR7, CXC chemokine receptor type (CXCR)4, CXCR5, and CXCR6 are expressed by MSCs isolated from human adipose tissue [2].
Inflammatory cytokines play an important role in the pathophysiology of SAP. TNF-α, IL-1β, and IL-6 are pro-inflammatory cytokines released predominantly by innate immune cells during SAP [440] that activate and induce the differentiation of T cells [14]. T helper type 1 (Th1) and Th17 cells produce the pro-inflammatory cytokines IFN-γ, IL-12, IL-17, and IL-23, and their numbers are increased during SAP [19]. MSCs derived from human tissues have the ability to suppress pro-inflammatory cytokine expression [1619202837]. In this study, migrated cAT-MSCs inhibited the expression of pro-inflammatory cytokines, whereas the levels of anti-inflammatory cytokines such as IL-4 and IL-10, which are mainly released by Th2 cells and can reduce SAP [2535], were upregulated in the injured pancreas. The anti-inflammatory effects of cAT-MSC infusion were supported by the observed decrease and increase in serum concentrations of IFN-γ and IL-10, respectively.
Our results suggest that infused cAT-MSCs improve SAP in rats by inhibiting pro-inflammatory cytokines and stimulating anti-inflammatory cytokine production. Previous in vitro studies have shown that canine MSCs suppress the proliferation of T cells that release inflammatory cytokines and mediators [622]. Furthermore, FoxP3+ regulatory T cells induced by co-culture with MSCs are known to induce apoptosis in innate immune cells and CD4+ T cells [931]. In the present study, cAT-MSCs suppressed the proliferation of co-cultured cPBMCs as well as rat splenocytes treated with ConA in a ratio-dependent manner. Recent studies have also shown that MSCs release soluble factors that have anti-inflammatory effects [33]. The TNF-inducible protein TSG-6, which is a major soluble factor released by MSCs, not only suppressed macrophages and T cells, but also stimulated the production of FoxP3+ regulatory T cells in damaged tissues [101224]. Similarly, CM containing soluble factors inhibited the proliferation of both cPBMCs and rat splenocytes in the present study. In addition, cAT-MSCs blocked the infiltration of CD3+ T cells and increased the FoxP3+ regulatory T cell population in the injured pancreas of SAP rats. Although the identity of the soluble factors and anti-inflammatory mechanisms of cAT-MSCs require more detailed study, we speculate that cAT-MSCs inhibit inflammation by regulating T cells via paracrine mechanisms as well as cell-to-cell contact based on previous studies and the results of the present study.
In conclusion, the results presented herein demonstrate that cAT-MSCs can improve pancreatic injury and regulate inflammatory cytokines by inducing FoxP3+ regulatory T cells and suppressing T cell proliferation in rats with SAP. Therefore, we suggest that cAT-MSCs are an attractive candidate for cell-based clinical therapy in SAP dogs.
Figures and Tables
Fig. 1
Identification of mesenchymal stem cells (MSCs) isolated from canine adipose tissue. (A) Immunophenotypic analysis by flow cytometry. (B) Adipogenic, osteogenic, and chondrogenic differentiation of canine adipose tissue-derived (cAT)-MSCs. 200×. Scale bars = 20 µm (B).
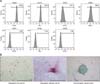
Fig. 2
Therapeutic effects of cAT-MSCs in rats with severe acute pancreatitis (SAP). (A) Histopathological analysis. (B) Serum amylase activities (U/L). (C) Lipase activities (U/L). (D) Pancreas to body weight ratio. Data are shown as the means ± standard deviation (SD). *p < 0.05, ***p < 0.001. Scale bars = 20 µm (A).
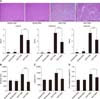
Fig. 3
Tracking of infused cAT-MSCs. (A) Pancreatic tissue sections following systemic administration of CM-DiI-labeled cAT-MSCs in rats with or without SAP. (B) PCR amplification of cRPS5 in pancreatic tissue. Lanes 1 and 2, SHAM+PBS; Lanes 3 and 4, SHAM+MSC; Lanes 5 and 6, SAP+PBS; Lanes 7 and 8; SAP+MSC; Lane 9, canine DNA. 200× (A).
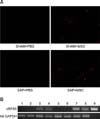
Fig. 4
Effect of cAT-MSCs on inflammatory cytokines levels. (A) mRNA expression of pro- and anti-inflammatory cytokines in pancreas tissue. (B) Serum levels of IFN-γ and IL-10. Data are shown as the means ± SD. *p < 0.05, **p < 0.01.

Fig. 5
T cell regulation by cAT-MSCs. cAT-MSCs suppressed the proliferation of cPBMCs (A) and rat splenocytes (B) stimulated with ConA. Detection of CD3+ T cells (C) and FoxP3+ regulatory T cells (D) in pancreatic tissue. Percentages of CD3+ or FoxP3+ cells are shown as the means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. 200× (C and D).

Acknowledgments
This study was supported by the Research Institute for Veterinary Science and BK21 PLUS Program for Creative Veterinary Science Research, Seoul National University. Korea.
References
1. Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007; 56:1175–1186.

2. Baek SJ, Kang SK, Ra JC. In vitro migration capacity of human adipose tissue-derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors. Exp Mol Med. 2011; 43:596–603.

4. Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000; 190:117–125.

5. Brinkhof B, Spee B, Rothuizen J, Penning LC. Development and evaluation of canine reference genes for accurate quantification of gene expression. Anal Biochem. 2006; 356:36–43.

6. Carrade DD, Borjesson DL. Immunomodulation by mesenchymal stem cells in veterinary species. Comp Med. 2013; 63:207–217.
7. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007; 25:2739–2749.

8. Cook A, Breitschwerdt EB, Levine JF, Bunch SE, Linn LO. Risk factors associated with acute pancreatitis in dogs: 101 cases (1985-1990). J Am Vet Med Assoc. 1993; 203:673–679.
9. English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E2 and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25Highforkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009; 156:149–160.

10. François M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012; 20:187–195.

11. González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009; 136:978–989.

12. Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009; 58:929–939.

13. Hess RS, Saunders HM, Van Winkle TJ, Shofer FS, Washabau RJ. Clinical, clinicopathologic, radiographic, and ultrasonographic abnormalities in dogs with fatal acute pancreatitis: 70 cases (1986-1995). J Am Vet Med Assoc. 1998; 213:665–670.
14. Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993; 260:547–549.

15. Hu J, Zhang L, Wang N, Ding R, Cui S, Zhu F, Xie Y, Sun X, Wu D, Hong Q, Li Q, Hi S, Liu X, Chen X. Mesenchymal stem cells attenuate ischemic acute kidney injury by inducing regulatory T cells through splenocyte interactions. Kidney Int. 2013; 84:521–531.

16. Hua J, He ZG, Qian DH, Lin SP, Gong J, Meng HB, Yang TS, Sun W, Xu B, Zhou B, Song ZS. Angiopoietin-1 gene-modified human mesenchymal stem cells promote angiogenesis and reduce acute pancreatitis in rats. Int J Clin Exp Pathol. 2014; 7:3580–3595.
17. Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK Jr, Amann ST, Toskes PP, Liddle R, McGrath K, Uomo G, Post JC, Ehrlich GD. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996; 14:141–145.

18. Jung KH, Shin HP, Lee S, Lim YJ, Hwang SH, Han H, Park HK, Chung JH, Yim SV. Effect of human umbilical cord blood-derived mesenchymal stem cells in a cirrhotic rat model. Liver Int. 2009; 29:898–909.

19. Jung KH, Song SU, Yi T, Jeon MS, Hong SW, Zheng HM, Lee HS, Choi MJ, Lee DH, Hong SS. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology. 2011; 140:998–1008.

20. Jung KH, Yi T, Son MK, Song SU, Hong SS. Therapeutic effect of human clonal bone marrow-derived mesenchymal stem cells in severe acute pancreatitis. Arch Pharm Res. 2015; 38:742–751.

21. Kallmeyer K, Pepper MS. Homing properties of mesenchymal stromal cells. Expert Opin Biol Ther. 2015; 15:477–479.

22. Kang JW, Kang KS, Koo HC, Park JR, Choi EW, Park YH. Soluble factors-mediated immunomodulatory effects of canine adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2008; 17:681–693.

23. Kehoe O, Cartwright A, Askari A, El Haj AJ, Middleton J. Intra-articular injection of mesenchymal stem cells leads to reduced inflammation and cartilage damage in murine antigen-induced arthritis. J Transl Med. 2014; 12:157.

24. Kota DJ, Wiggins LL, Yoon N, Lee RH. TSG-6 produced by hMSCs delays the onset of autoimmune diabetes by suppressing Th1 development and enhancing tolerogenicity. Diabetes. 2013; 62:2048–2058.

25. Kusske AM, Rongione AJ, Ashley SW, McFadden DW, Reber HA. Interleukin-10 prevents death in lethal necrotizing pancreatitis in mice. Surgery. 1996; 120:284–289.

26. Li H, Yan F, Lei L, Li Y, Xiao Y. Application of autologous cryopreserved bone marrow mesenchymal stem cells for periodontal regeneration in dogs. Cells Tissues Organs. 2009; 190:94–101.

27. Mansfield C. Acute pancreatitis in dogs: advances in understanding, diagnostics, and treatment. Top Companion Anim Med. 2012; 27:123–132.

28. Meng HB, Gong J, Zhou B, Hua J, Yao L, Song ZS. Therapeutic effect of human umbilical cord-derived mesenchymal stem cells in rat severe acute pancreatitis. Int J Clin Exp Pathol. 2013; 6:2703–2712.
29. Moodley Y, Atienza D, Manuelpillai U, Samuel CS, Tchongue J, Ilancheran S, Boyd R, Trounson A. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol. 2009; 175:303–313.

30. Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg. 1998; 175:76–83.

31. Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation–mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007; 8:1353–1362.

32. Penha EM, Meira CS, Guimarães ET, Mendonça MV, Gravely FA, Pinheiro CM, Pinheiro TM, Barrouin-Melo SM, Ribeiro-Dos-Santos R, Soares MB. Use of autologous mesenchymal stem cells derived from bone marrow for the treatment of naturally injured spinal cord in dogs. Stem Cells Int. 2014; 2014:437521.

34. Tsuda H, Yamahara K, Otani K, Okumi M, Yazawa K, Kaimori JY, Taguchi A, Kangawa K, Ikeda T, Takahara S, Isaka Y. Transplantation of allogenic fetal membrane-derived mesenchymal stem cells protects against ischemia/reperfusion-induced acute kidney injury. Cell Transplant. 2014; 23:889–899.

35. Van Laethem JL, Marchant A, Delvaux A, Goldman M, Robberecht P, Velu T, Devière J. Interleukin 10 prevents necrosis in murine experimental acute pancreatitis. Gastroenterology. 1995; 108:1917–1922.

36. Vieira NM, Brandalise V, Zucconi E, Secco M, Strauss BE, Zatz M. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant. 2010; 19:279–289.

37. Yang B, Bai B, Liu CX, Wang SQ, Jiang X, Zhu CL, Zhao QC. Effect of umbilical cord mesenchymal stem cells on treatment of severe acute pancreatitis in rats. Cytotherapy. 2013; 15:154–162.

38. Yu X, Lu C, Liu H, Rao S, Cai J, Liu S, Kriegel AJ, Greene AS, Liang M, Ding X. Hypoxic preconditioning with cobalt of bone marrow mesenchymal stem cells improves cell migration and enhances therapy for treatment of ischemic acute kidney injury. PloS One. 2013; 8:e62703.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download