Abstract
The continuous exposure of cats to diverse influenza viruses raises the concern of a potential role of cats in the epidemiology of these viruses. Our previous seroprevalence study of domestic cat sera collected during the 2009 H1N1 pandemic wave (September 2009–September 2010) revealed a high prevalence of pandemic H1N1, as well as seasonal H1N1 and H3N2 human flu virus infection (22.5%, 33.0%, and 43.5%, respectively). In this study, we extended the serosurvey of influenza viruses in cat sera collected post-pandemic (June 2011–August 2012). A total of 432 cat sera were tested using the hemagglutination inhibition assay. The results showed an increase in pandemic H1N1 prevalence (33.6%) and a significant reduction in both seasonal H1N1 and H3N2 prevalence (10.9% and 17.6%, respectively) compared to our previous survey conducted during the pandemic wave. The pandemic H1N1 prevalence in cats showed an irregular seasonality pattern in the post-pandemic phase. Pandemic H1N1 reactivity was more frequent among female cats than male cats. In contrast to our earlier finding, no significant association between clinical respiratory disease and influenza virus infection was observed. Our study highlights a high susceptibility among cats to human influenza virus infection that is correlated with influenza prevalence in the human population.
Influenza is a highly contagious viral infection caused by influenza A viruses that infect both humans and animals. Although influenza A viruses are generally host-specific, interspecies transmission has been reported among different species worldwide [39].
The susceptibility of cats to human H2N2 and H3N2 [1124], seal H7N7 and avian H7N3 viruses [11] was initially reported in the 1970s. Epidemiological studies of influenza in cats have been limited, and recent studies of influenza in cats have primarily focused on the highly pathogenic avian influenza (HPAI) H5N1 and H7N7, and more recently the 2009 pandemic H1N1 virus. Experimental infections have demonstrated that cats are susceptible to the (HPAI) H5N1 and H7N7 [335] and the 2009 pandemic H1N1 [33] influenza viruses. Infected cats developed respiratory signs and lesions that were similar to those observed under natural circumstances, and viral shedding occurred via the respiratory and digestive tracts. Both natural and experimental infections of influenza viruses in cats support the possibility of lateral transmission [2833] and adaptation of these viruses, with a concern of evolution to more virulent strains for cats and other mammalian species.
Though susceptibility of cats to the human H3N2 subtype was noted in the early 1970s with serological evidence of the human H3N2 subtype infection in cats during human epidemics [1018], no further research was pursued to investigate the prevalence of human influenza virus subtypes in cats until recently [11025]. Moreover, recent reports showed that cats are susceptible to low pathogenic avian influenza viruses such as H1N9 and H4N6 subtypes isolated from shorebirds and to avian H9N2 viruses [72840]. Interspecies transmission of canine influenza H3N2 virus was also reported in cats [121328]. Infections with low pathogenic avian influenza strains were associated with lung lesions and shedding of viruses without clinical signs. These findings indicate a potential role of cats as a source of infection to other animals and human beings. It is also worth noting that recent reports highlighted the efficient replication of low pathogenic avian influenza viruses in wild-caught house mice without adaptation [27]. The transmission of such viruses to cats via ingestion of infected mice, similar to that observed upon ingestion of HPAI H5N1 infected bird carcasses [23], is another possible trans-species pathway of influenza virus spread.
Serological surveys conducted in Italy using nucleoprotein-specific competitive enzyme-linked immunosorbent assay (ELISA) to screen cat sera collected from 1999 to 2005 [17], and sera collected from 2004 to 2008 [21] showed no evidence of influenza antibodies. On the other hand, recent serosurveys using ELISA indicated the presence of influenza specific antibodies in the sera of domestic cats, although the prevalence was low (1.5–2.5%) [4691330]. The differences in sample collection time and exposure, as well as the poor repeatability and low sensitivity of the NP-based ELISA [1] may explain the low prevalence or failure to detect influenza infection in previous studies using different ELISA assays.
Serosurveys using hemagglutination inhibition (HI) assay during 1997–2008 in Japan and the United States [115] demonstrated a higher prevalence of seasonal human H3N2 influenza viruses than earlier studies using ELISA. Moreover, a surprisingly high prevalence of the 2009 pandemic H1N1 influenza virus was detected in cat sera collected during and after the pandemic wave than in the pre-pandemic period [115]. Considering the high sensitivity and specificity of the HI test [5], these findings provide clear evidence of routine exposure of cats to human influenza viruses. The unpredictable nature of influenza viruses together with the paucity of both experimental infection and seroprevalence studies in cats have made it difficult to determine their potential role in the epidemiology of influenza A viruses.
In this study, we extended our previous serosurvey [1] of seasonal and pandemic human influenza infection in cats to assess seroprevalence in the post-pandemic phase starting from the 2011 spring season to the summer season of 2012 (June 2011 to August 2012). The data were analyzed based on the collection date, sex, age, and health status of the tested cats at the time of sampling.
Serum samples (n = 432) were collected between June 2011 and August 2012 from domestic cats that presented to the Ohio State University Veterinary Medical Center. Date of sample collection, sex, age, and presenting complaint were obtained from the medical history. Fig. 1 shows number of samples collected based on sex and age. In this study, cats that presented to the clinic with complaints unrelated to the respiratory system accounted for 57.6% of the samples, including gastrointestinal, cardiac, urinary, tumor and metabolic disorders. The remaining cats were those that presented with respiratory signs (9.1%) and for wellness care (33.3%). When ≥ 2 samples from the same cat were obtained, they were considered independent if they remained negative with at least a 30 day interval. For positive samples, subsequent samples from the same animal were not counted when determining the prevalence rate.
Pandemic H1N1 (A/OH/0925-1/09), seasonal H1N1 (A/Ohio/K1130/06) and H3N2 (A/human/Ohio/06) viruses were obtained from the Ohio Department of Health (Reynoldsburg, OH). Stocks of the viruses were prepared in eggs or Madin-Darby canine kidney (MDCK) cells and used in the HI and virus neutralization assays described below. Positive control sera against pandemic H1N1 and seasonal H1N1 and H3N2 viruses were previously prepared in our laboratory [1].
The hemagglutination inhibition (HI) test was carried out according to the World Organization for Animal Health Manual [38] with minor modification. Briefly, all sera were heat inactivated at 56℃ for 30 min. We previously found no significant difference in HI titers between serum samples treated with receptor-destroying enzyme (RDE) and untreated samples [1]. Thus, samples were not treated with RDE to avoid initial dilution of the serum samples. Two-fold serial dilutions of sera were mixed with an equal amount of 8 hemagglutination units (HAU) of each virus, after which the mixture was incubated at room temperature for 30 min. The HI reactivity was determined by addition of 1% turkey red blood cells. A cut-off value of ≥ 4 log2 (1 : 16) HI titers was considered seropositive for any of the tested influenza subtypes. A subset of HI positive sera was confirmed by the virus neutralization (VN) test. Six positive serum samples for each subtype with HI titers ≥ 40 were included. The VN test was performed as previously described [31], and the titers were expressed as the reciprocal of the highest serum dilution giving complete inhibition of the virus growth.
To determine the relationship between patient demographics and seropositivity as the response variable, univariable and multivariable linear and logistic regression and multinomial logistic regression models were used. The outcome (i.e., seropositivity) was a binary variable, while predictors of seropositivity were either dichotomous variables (sex), continuous variables (temperature and age), or categorical variables (health status). Analyses were conducted using the STATA (ver. 10.1) software (StataCorp, USA). The Spearman rank correlation coefficient was calculated to estimate the correlation between the HI and VN titers. One way ANOVA was used to determine the significance of differences (p < 0.05) between the log2 HI antibody titers against three tested strains.
The post-pandemic seroprevalence of pandemic H1N1, and the seasonal H1N1 and H3N2 viruses is shown in Fig. 2. The pandemic H1N1 showed higher prevalence of 33.6%; however, the prevalence of both seasonal H1N1 and H3N2 were significantly reduced to 10.9% and 17.6%, respectively, compared to seroprevalence during the pandemic wave (22.5%, 33.0%, and 43.5% for pandemic H1N1, seasonal H1N1, and H3N2, respectively). The average log2HI titers against the seasonal H3N2, H1N1 and pandemic H1N1 subtypes were 4.3 ± 0.5, 5.3 ± 1.3 and 5.6 ± 1.3, respectively (Fig. 3). The HI titers against the pandemic H1N1 were significantly higher than those of the seasonal H3N2 viruses, but the difference between the pandemic and seasonal H1N1 viruses was not significant. Of the 432 total serum samples tested, 232 cats (53.7%) were negative for all three influenza virus subtypes tested and 144 (32.9%) were positive for one of the influenza virus subtypes tested, while 49 (11.1%) and 10 (2.3%) samples were concurrently positive for two and three influenza virus subtypes, respectively. The HI antibody titers were confirmed to be subtype specific as indicated by a positive correlation of both HI and VN antibody titers (Spearman correlation coefficient 0.52, p = 0.01).
Similar to our previous observations [1], statistical analysis showed that age remains a non-significant predictor for seropositivity of any of the subtypes of influenza tested. Though not statistically significant, we observed a higher prevalence of seasonal H3N2 influenza in geriatric cats aged above 15 years than to other influenza subtypes tested (Fig. 4).
As shown in Fig. 2 and indicated by multivariable analyses using the seropositivity as binary data (influenza virus positive/negative) to determine the association between variables of interest and the probability of testing positive to influenza virus, no significant association was observed between the average monthly temperature (i.e. seasonality) and the probability of being seropositive to at least one influenza virus subtype. However, seropositivity increased during the winter and spring seasons of 2012. We also observed an increased prevalence of the pandemic H1N1 in the late spring and summer seasons of 2012 (Fig. 2).
When compared to the serosurvey we conducted on cat sera during and right after the pandemic wave in humans, gender was shown to be a significant predictor of the pandemic H1N1 seropositivity during the post-pandemic period, with female cats having higher probability to test positive for influenza viruses than male cats (OR = 1.9, 95% CI = 1.4–5.3, and logistic p = 0.05). Univariable analysis indicated that the health status of the cats is a non-significant predictor for influenza virus seropositivity of any subtype (logistic p > 0.2). However, it should be noted that the majority of cats tested in this study presented for wellness care or with non-respiratory signs (about 91%), while only a small number of samples were from cats with signs of respiratory disease (9.1%) (Table 1).
The infection of domestic cats with influenza viruses of different origins, including avian, and recently with the 2009 pandemic H1N1 has been reported. These reports along with the history of close contact of cats with the infected humans or birds in most of the reported cases [82933] have raised the concern that companion animals may act as host species contributing to the adaptation of avian viruses in mammals as well as a potential reservoir of mammalian influenza viruses to human beings. There have been several serosurveillance studies conducted in the United States, but few have indicated a high prevalence of human influenza virus infection in cats [115].
To investigate possible changes in the prevalence of the seasonal human and pandemic H1N1 influenza viruses in cats, especially after the pandemic H1N1 wave, we extended the serosurvey of human influenza virus infection in cat sera collected during the post-pandemic wave between the spring of 2011 and the summer of 2012. When compared to the situation in human beings, in which the 2009 pandemic H1N1 virus was the predominant virus during the 2010–2011 post-pandemic season and then drastically decreased in the 2011–2012 season [19], the prevalence of pandemic H1N1 in cats were higher (33.6%) than those we reported during and immediately after the pandemic wave in the same geographical region [1]. Moreover, the prevalence of seasonal H1N1 and H3N2 (10.9 and 17.6%, respectively) were significantly lower than previously reported [115].
The low prevalence of the seasonal H1N1 and H3N2 human influenza viruses observed in the current study indicate that the dominance of 2009 pandemic H1N1 over the seasonal flu viruses in the post-pandemic wave that was seen in humans may be reflected in cats because there is more frequent pandemic H1N1 virus transmission from humans [16]. This competitive advantage may be because cats are naïve to pandemic H1N1, while many cats have some immunity to seasonal flu viruses. Differences in the receptor binding pattern of pandemic H1N1 and seasonal influenza viruses seen in vitro may reflect the susceptibility of the cats [1434]. A similar trend of pandemic H1N1 dominance was also observed in humans during the 2009–2010 and 2010–2011 influenza seasons, which was suggested to be due to cross-protective immune response specific for the pandemic H1N1 stalk region, which is highly conserved in the two H1N1 strains [16]. Additionally, antibodies against the same subtype of neuraminidase protein in both strains may have further reduced the spread of the less virulent seasonal H1N1 strain [1620].
Similar to the prevalence during and immediately after the pandemic H1N1 wave in cats, pandemic H1N1 in cats seems to be irregular without showing typical seasonality pattern of influenza in humans. There was an increase in pandemic H1N1 prevalence during the late spring and the summer seasons of 2012, which may be explained by previous exposure to the virus during the spring season that passed undetected considering the high prevalence we reported in late spring of 2012, as well as the high prevalence in healthy cats [1].
In contrast to our previous survey in cats [1], non-significant association of both seasonal H1N1 and H3N2 prevalence with the lower ambient temperatures in the post-pandemic cat sera was observed. Factors that influence the association of the prevalence of seasonal influenza viruses may include the overwhelming prevalence of pandemic H1N1 that does not follow seasonality and the fact that about 50% of samples included in this study were collected during the summer. Moreover, the average temperatures in central Ohio during the period between November 2011 and March 2012 (i.e., the cold season) were elevated by about 0.9℃ to 5.5℃ (National Centers for Environmental Information, USA) relative to the temperatures reported at the same time during our previous surveillance study conducted in 2009–2010.
Statistical analysis also demonstrated that female cats showed higher susceptibility to pandemic H1N1 than male cats. Epidemiological studies in humans, such as those conducted in Thailand [32], Rwanda [36] and the United States [26] also showed that about 60% of confirmed human cases of pandemic H1N1 were in females. This association may be attributed to the high prevalence of seasonal H3N2 observed in samples collected from female cats, especially those more than 15 years of age (i.e., geriatric). The increased risk of pandemic H1N1 infection and hospitalization in female human beings was attributed to several physiologic and hormonal changes that may alter functionality of the cardiovascular and respiratory systems [2237]. However, it is not possible to determine if such changes also apply to female cats in this study, because the vast majority of female cats in the study had been routinely spayed (ovariohysterectomized).
There was no significant association observed between the respiratory illness and any of the influenza subtypes tested. It is likely that the diseased animals showed clinical signs in the acute phase before antibody titer mount. Similar observations were reported in humans, especially with the pandemic H1N1, where 54.4% of seropositive individuals did not experience influenza-like illness [26]. However, the smaller sample size with respiratory signs may have restricted the ability of the statistical model we used to assess such associations. The non-significant association between age and influenza viruses in cats further supports our previous assumption that the shorter life span of cats and the lack of previous exposure to the influenza viruses that are antigenically related to the currently circulating influenza viruses [1] hinder assessment of a possible effect of age on the prevalence of influenza viruses in cats.
In conclusion, our results showed increased seroprevalence of pandemic H1N1 in post-pandemic cat sera over seasonal influenza viruses. Although the epidemiology of the 2009 pandemic H1N1 in cats is similar to that observed in humans with a one year delay, we cannot conclude if this is related to exposure due to human to cat transmission or establishment of the pandemic H1N1 influenza virus in feline population. The virus surveillance along with the serological surveillance studies could help understand future patterns of human influenza viruses in cats. These findings in addition to the increased reports of the susceptibility of cats to avian, human, and other mammalian influenza viruses indicate that cats are an additional influenza host of public health importance.
Figures and Tables
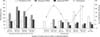 | Fig. 2Post-pandemic seroprevalence of human influenza viruses in domestic cats relative to seasonal average ambient temperature in Ohio, the United States. |
 | Fig. 3Average Log2 HI antibody titer against pandemic H1N1 and seasonal H1N1 and H3N2 influenza viruses. Pandemic H1N1 HI antibody titers were significantly higher than those of the seasonal H3N2 virus (***p < 0.001). |
Acknowledgments
The authors thank Megan Strother for technical assistance. This research was partly supported by the National Institutes of Health (R21AI077787) and the Ohio Agricultural Research and Development Center Research Program of the United States.
References
1. Ali A, Daniels JB, Zhang Y, Rodriguez-Palacios A, Hayes-Ozello K, Mathes L, Lee CW. Pandemic and seasonal human influenza virus infections in domestic cats: prevalence, association with respiratory disease, and seasonality patterns. J Clin Microbiol. 2011; 49:4101–4105.

2. Campagnolo ER, Rankin JT, Daverio SA, Hunt EA, Lute JR, Tewari D, Acland HM, Ostrowski SR, Moll ME, Urdaneta VV, Ostroff SM. Fatal pandemic (H1N1) 2009 influenza A virus infection in a Pennsylvania domestic cat. Zoonoses Public Health. 2011; 58:500–507.

3. Chang S, Ding Z, Yang ST, Gao YW, Zou XH, Wang TC, Xia XZ. Study on the histopathology of cats inoculated with H5N1 subtype high pathogenic avian influenza virus originated from tigers. Bing Du Xue Bao. 2007; 23:477–480.
4. Chen Y, Zhang J, Qiao C, Yang H, Zhang Y, Xin X, Chen H. Co-circulation of pandemic 2009 H1N1, classical swine H1N1 and avian-like swine H1N1 influenza viruses in pigs in China. Infect Genet Evol. 2013; 13:331–338.

5. Comin A, Toft N, Stegeman A, Klinkenberg D, Marangon S. Serological diagnosis of avian influenza in poultry: is the haemagglutination inhibition test really the 'gold standard'? Influenza Other Respir Viruses. 2013; 7:257–264.

6. Damiani AM, Kalthoff D, Beer M, Müller E, Osterrieder N. Serological survey in dogs and cats for influenza A(H1N1)pdm09 in Germany. Zoonoses Public Health. 2012; 59:549–552.

7. Driskell EA, Jones CA, Berghaus RD, Stallknecht DE, Howerth EW, Tompkins SM. Domestic cats are susceptible to infection with low pathogenic avian influenza viruses from shorebirds. Vet Pathol. 2013; 50:39–45.

8. Fiorentini L, Taddei R, Moreno A, Gelmetti D, Barbieri I, De Marco MA, Tosi G, Cordioli P, Massi P. Influenza A pandemic (H1N1) 2009 virus outbreak in a cat colony in Italy. Zoonoses Public Health. 2011; 58:573–581.

9. Gordy JT, Jones CA, Rue J, Crawford PC, Levy JK, Stallknecht DE, Tripp RA, Tompkins SM. Surveillance of feral cats for influenza A virus in north central Florida. Influenza Other Respir Viruses. 2012; 6:341–347.

10. Harder TC, Vahlenkamp TW. Influenza virus infections in dogs and cats. Vet Immunol Immunopathol. 2010; 134:54–60.

11. Hinshaw VS, Webster RG, Easterday BC, Bean WJ Jr. Replication of avian influenza A viruses in mammals. Infect Immun. 1981; 34:354–361.

12. Jeoung HY, Lim SI, Shin BH, Lim JA, Song JY, Song DS, Kang BK, Moon HJ, An DJ. A novel canine influenza H3N2 virus isolated from cats in an animal shelter. Vet Microbiol. 2013; 165:281–286.

13. Jeoung HY, Shin BH, Lee WH, Song DS, Choi YK, Jeong W, Song JY, An DJ. Seroprevalence of subtype H3 influenza A virus in South Korean cats. J Feline Med Surg. 2012; 14:746–750.

14. Löhr CV, DeBess EE, Baker RJ, Hiett SL, Hoffman KA, Murdoch VJ, Fischer KA, Mulrooney DM, Selman RL, Hammill-Black WM. Pathology and viral antigen distribution of lethal pneumonia in domestic cats due to pandemic (H1N1) 2009 influenza A virus. Vet Pathol. 2010; 47:378–386.

15. McCullers JA, Van De Velde LA, Schultz RD, Mitchell CG, Halford CR, Boyd KL, Schultz-Cherry S. Seroprevalence of seasonal and pandemic influenza A viruses in domestic cats. Arch Virol. 2011; 156:117–120.

16. Palese P, Wang TT. Why do influenza virus subtypes die out? A hypothesis. MBio. 2011; 2:e00150-11.

17. Paltrinieri S, Spagnolo V, Giordano A, Martin AM, Luppi A. Influenza virus type A serosurvey in cats. Emerg Infect Dis. 2007; 13:662–664.

18. Paniker CKJ, Nair CMG. Infection with A2 Hong Kong influenza virus in domestic cats. Bull World Health Organ. 1970; 43:859–862.
19. Pariani E, Amendola A, Ranghiero A, Anselmi G, Zanetti A. Surveillance of influenza viruses in the post-pandemic era (2010-2012) in Northern Italy. Hum Vaccin Immunother. 2013; 9:657–666.

20. Pica N, Hai R, Krammer F, Wang TT, Maamary J, Eggink D, Tan GS, Krause JC, Moran T, Stein CR, Banach D, Wrammert J, Belshe RB, García-Sastre A, Palese P. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci U S A. 2012; 109:2573–2578.

21. Piccirillo A, Pasotto D, Martin AM, Cordioli P. Serological survey for influenza type A viruses in domestic dogs (Canis lupus familiaris) and cats (Felis catus) in north-eastern Italy. Zoonoses Public Health. 2010; 57:239–243.

22. Rasmussen SA, Jamieson DJ, Uyeki TM. Effects of influenza on pregnant women and infants. Am J Obstet Gynecol. 2012; 207:3 Suppl. S3–S8.

23. Rimmelzwaan GF, van Riel D, Baars M, Bestebroer TM, van Amerongen G, Fouchier RAM, Osterhaus ADME, Kuiken T. Influenza A virus (H5N1) infection in cats causes systemic disease with potential novel routes of virus spread within and between hosts. Am J Pathol. 2006; 168:176–183.

24. Romváry J, Rózsa J, Farkas E. Infection of dogs and cats with the Hong Kong influenza A (H3N2) virus during an epidemic period in Hungary. Acta Vet Acad Sci Hung. 1975; 25:255–259.
25. Said AW, Usui T, Shinya K, Ono E, Ito T, Hikasa Y, Matsuu A, Takeuchi T, Sugiyama A, Nishii N, Yamaguchi T. A sero-survey of subtype H3 influenza A virus infection in dogs and cats in Japan. J Vet Med Sci. 2011; 73:541–544.

26. Shafir SC, O'Keefe KA, Shoaf KI. Evaluation of the seroprevalence of influenza A(H1N1) 2009 on a university campus: a cross-sectional study. BMC Public Health. 2011; 11:922.

27. Shriner SA, VanDalen KK, Mooers NL, Ellis JW, Sullivan HJ, Root JJ, Pelzel AM, Franklin AB. Low-pathogenic avian influenza viruses in wild house mice. PLoS One. 2012; 7:e39206.

28. Song DS, An DJ, Moon HJ, Yeom MJ, Jeong HY, Jeong WS, Park SJ, Kim HK, Han SY, Oh JS, Park BK, Kim JK, Poo H, Webster RG, Jung K, Kang BK. Interspecies transmission of the canine influenza H3N2 virus to domestic cats in South Korea, 2010. J Gen Virol. 2011; 92:2350–2355.

29. Songserm T, Amonsin A, Jam-on R, Sae-Heng N, Meemak N, Pariyothorn N, Payungporn S, Theamboonlers A, Poovorawan Y. Avian influenza H5N1 in naturally infected domestic cat. Emerg Infect Dis. 2006; 12:681–683.

30. Su S, Yuan L, Li H, Chen J, Xie J, Huang Z, Jia K, Li S. Serologic evidence of pandemic influenza virus H1N1 2009 infection in cats in China. Clin Vaccine Immunol. 2013; 20:115–117.

31. Swayne DE, Senne DA, Beard CW. Avian influenza. In : Swayne DE, editor. A Laboratory Manual for the Isolation and Identification of Avian Pathogens. 4th ed. Kennett Square: American Association of Avian Pathologists;1998. p. 150–155.
32. Unsematham S, Ketbumrongporn W, Boonthongtho K. Associated factors for diagnosis of influenza A (H1N1) 2009 in patients presenting to Rajavithi Hospital. J Med Assoc Thai. 2012; 95:Suppl 3. S16–S21.
33. van den Brand JM, Stittelaar KJ, van Amerongen G, van de Bildt MW, Leijten LM, Kuiken T, Osterhaus AD. Experimental pandemic (H1N1) 2009 virus infection of cats. Emerg Infect Dis. 2010; 16:1745–1747.

34. van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, Kuiken T. Human and avian influenza viruses target different cells in the lower respiratory tract of humans and other mammals. Am J Pathol. 2007; 171:1215–1223.

35. van Riel D, Rimmelzwaan GF, van Amerongen G, Osterhaus ADME, Kuiken T. Highly pathogenic avian influenza virus H7N7 isolated from a fatal human case causes respiratory disease in cats but does not spread systemically. Am J Pathol. 2010; 177:2185–2190.

36. Wane J, Nyatanyi T, Nkunda R, Rukelibuga J, Ahmed Z, Biedron C, Kabeja A, Muhimpundu MA, Kabanda A, Antara S, Briet O, Koama JB, Rusanganwa A, Mukabayire O, Karema C, Raghunathan P, Lowrance D. 2009 pandemic influenza A (H1N1) virus outbreak and response—Rwanda, October, 2009-May, 2010. PLoS One. 2012; 7:e31572.
37. Ward KA, Spokes PJ, McAnulty JM. Case–control study of risk factors for hospitalization caused by pandemic (H1N1). Emerg Infect Dis. 2011; 17:1409–1416.
38. World Organisation for Animal Health. Avian influenza. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Mammals, Birds and Bees). 6th ed. Vol. 1. Paris: Office International des Epizooties;2008. p. 465–481.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download