Abstract
Mycobacterium (M.) vaccae is a fast-growing species of saprophytic bacteria that is widely distributed. To understand the host immune responses induced by M. vaccae isolated from bovine submaxillary lymph nodes, C57BL/6 mice were infected with reference strain M. vaccae Bacillus Calmette-Guérin (BCG) and isolated M. vaccae using intraperitoneal injections. Comparison of the bacterial replication and organ pathology between M. vaccae and M. vaccae BCG revealed that M. vaccae was more malignant than M. vaccae in mice. We also demonstrated that serum from the M. vaccae-infected mice contained a higher expression level of gamma-interferon (IFN-γ), tumor necrosis factor alpha, monocyte chemoattractant protein-1, interleukin (IL)-4, IL-12, IL-10 and transforming growth factor beta than did the other groups, especially after week 4. Furthermore, when the numbers of CD3+CD4+IFN-γ+ and CD3+CD4+IL4+ cells in the infected mice were observed by flow cytometry, we found that a powerful T helper 1 (Th1) response was induced by M. vaccae infection, which was associated with the emergence of CD3+CD4+IFN-γ+ cells. However, the Th1 response declined over time, which was associated with appearance of the CD4+CD25+FoxP3+ and CD4+CD25+CD152+Treg cell reaction. In addition, a strong Th2 response was found. Finally, we found that M. vaccae infection increased the production of type I IFNs, which was associated with a reduced Th1 response.
Mycobacterial species other than those belonging to the Mycobacterium (M.) tuberculosis complex or M. leprae are known as nontuberculous mycobacteria (NTM), environmental mycobacteria, or atypical mycobacteria [14]. NTM are ubiquitous in the environment and induce opportunistic infections that can produce a wide range of diseases, and a large number of NTM have been isolated from mammals [20]. As opportunists, NTM cause acute or chronic infections in patients with cystic fibrosis [1], cancer [4], HIV [21] and immunosuppression [18]. In humans, NTM comprise an important clinical problem associated with significant morbidity, and their occurrence is increasing worldwide [35]. Accordingly, researchers are searching for an immunogenetic factor that will illuminate susceptibility to non-tuberculous mycobacterial disease. To identify new therapeutic targets and enable rational vaccine design, increased understanding of the molecular and cellular host defense mechanisms providing protective immunity against NTM is needed [17]. However, most studies of NTM conducted to date have focused on epidemiological investigations [35], drug sensitivity [11] and laboratory diagnosis [33]. Indeed, there have been few investigations of host immune mechanisms following infection or the reasons for which NTM can persist in the host. Therefore, there is an urgent need to clarify the infection mechanisms of NTM.
Innate immune responses play an important role in bacterial destruction; however, the chronic nature and high incidence of M. tuberculosis with low levels of CD4+ T cells indicates the importance of adaptive immune effectors [1729]. T helper 1 (Th1) cell-mediated immune responses can effectively remove Mycobacterium [26]. Following infection, a central control step against mycobacteria is activation of CD4+ T helper 1 cells, which produce effector cytokines such as tumor necrosis factor alpha (TNF-α) and gamma-interferon (IFN-γ) [3]. The Th1 cytokines, such as interleukin (IL)-12, IL-23, IL-18 and TNF-α, are essential to inhibition of bacterial growth and restrained dissemination [1224]. An increased risk for disseminated NTM disease in humans includes defects in IL-12 [2], IFN-γ [10] or, more recently, interferon regulatory factor-8 (IRF-8) [16]. Some studies have suggested that the expression of immunosuppressive cytokines such as IL-10 and transforming growth factor beta (TGF-β) may be useful to inhibit infection with highly virulent bacterial strains, which are usually susceptible to these cytokines [9]. The active Th1 response and reduced Th2 responses in infected humans or mice protect the body [23]. Although type I IFNs are important to reducing the Th1 immunity during M. tuberculosis infection [25], no studies of type I IFNs during NTM infection have been conducted. Researchers have demonstrated a significant role of CD4+CD25+ regulatory T cells, which are associated with Th1 responses, during M. tuberculosis infection [27]. However, it is necessary to further investigate the function of CD4+ CD25+ regulatory T cells during NTM infection.
Most nosocomial NTM outbreaks are caused by rapidly growing mycobacteria [78], including M. vaccae. M. vaccae was first isolated and named by Bönicke and Juhasz [6] in 1963 using the Latin word for cattle (vaccae) because of its strong connection with cows. M. vaccae has been reported as a cause of clinical diseases, such as bovine nodular thelitis, in cattle [2232]. Moreover, this organism was found to be connected with soft tissue infections and pulmonary disease in four people at the University of Texas MD Anderson Cancer Center (Houston, USA) [15]. Studies have shown an immuno-protective effect of heat-killed M. vaccae on M. tuberculosis-infected mice. Heat-killed bacteria can induce a Th1 response, inhibit the effects of Th2 response, and increase the levels of CD4+T cells and the expression of cytokines [13]. However, few studies have investigated immune mechanisms during a live M. vaccae infection. In this study, we isolated a type of M. vaccae from bovine submaxillary lymph nodes in the Preventive Veterinary Laboratory (Jilin Agricultural University, China) [37]. The goal of this study was to compare the M. vaccae isolated from bovine submaxillary lymph nodes to M. bovis BCG in terms of their infection properties and host immune mechanisms.
M. bovis Bacillus Calmette-Guérin (BCG) was obtained from the Preventive Veterinary Laboratory (Jilin Agricultural University). M. vaccae was isolated from bovine submaxillary lymph nodes in the Preventive Veterinary Laboratory (Jilin Agricultural University) [37] and cultured in supplemented Middlebrook 7H9 Broth medium.
Specific pathogen-free (SPF) female C57BL/6 mice were bought from Beijing HFK Bioscience, China. Mice were used at six weeks of age. All of the experimental procedures complied with the requirements of the Animal Care and Ethics Committees of Jilin Agriculture University.
The mice were infected with reference strain M. bovis BCG and isolated M. vaccae using intraperitoneal injections of 1 × 106 bacilli per animal. At weeks 2, 4, 6 and 8, the bacterial load was measured by plating serial dilutions of organ homogenates onto supplemented Middlebrook 7H9 agar at 37℃, and the samples were enumerated one week later. A total of five animals were infected for each time point.
The lung samples were fixed in 10% formalin buffer for one week, after which the organs were sliced into 5 µm thick sections and stained using hematoxylin and eosin and acid-fast stains as previously described [25].
The cell suspensions were incubated with monoclonal antibodies at 4℃ for 30 min as previously described [2531]. Monoclonal antibodies against CD3 (clone 17A2, rat anti-mouse IgG), CD4 (clone GK1.5, rat anti-mouse IgG), CD8 (clone 53-6.7, rat anti-mouse IgG), CD25 (3C7, rat IgM), CD152 (cytotoxic T-lymphocyte-associated protein [CTLA]-4, cloneUC10-4F10-11, hamster anti-mouse IgG), CD11b (Mac-1, clone M1/70, rat anti-mouse IgG), CD11c (clone N418, anti-mouse IgG), CD45R/B220 (RA3-6B2, rat anti-mouse IgG), and Ly-6C/Gr1 (AL-21, rat anti-mouse IgG) (BD Biosciences). The monoclonal antibody data acquisition and analysis were performed by BD FACSCanto flow cytometry (BD Biosciences), and we examined the samples using a BD C6 flow cytometer (BD Biosciences).
The cells were stained as previously described and prepared for intracellular staining [2531]. The cell membranes were permeabilized according to the kit instructions (Fix/Perm kit; BD Pharmingen, USA), after which the cells were stained with antibodies against Foxp3 (MF23, rat anti-mouse IgG), IL-4 (24G2, rat anti-mouse IgG), and IFN-γ (XMG1.2, rat anti-mouse IgG; eBioscience, USA).
We collected mouse blood by removing the eyes, placing the blood in centrifuge tubes for 6 h at 37℃, and then centrifuging the samples for 10 min (1,509 × g). The supernatant serum was collected and frozen at -80℃ until analysis for inflammatory cytokines (IFN-γ, TNF-α, monocyte chemoattractant protein [MCP]-1,IL-4, IL-12, IL-10 and TGF-β) using an ELISA kit (BD Biosciences).
The bacterial load was quantified at weeks 2, 4, 6 and 8 after high-dose (1 × 106 colony-forming unit) intraperitoneal injection. Infection with M. vaccae and M. bovis BCG resulted in severe lung infection at 2 weeks, but the bacterial number gradually decreased and was completely cleared by week 8. The group infected with M. vaccae contained a larger number of bacteria than the M. bovis BCG group (p < 0.001; panel A in Fig. 1).
The lung histopathology was characterized in mice challenged with M. vaccae and M. bovis BCG (panels B and C in Fig. 1). M. vaccae–infected mice had inflammatory infiltrates with many acid-fast staining bacilli and large aggregates of lymphocytes (panel B in Fig. 1). At week 2, when a larger quantity of bacteria persisted, there was a local inflammatory response in the lungs. By weeks 4 and 6, the moderate-sized lesions had decreased in size. However, at week 8, when M. vaccae and M. bovis BCG strains were cleared, healthy lung tissue was observed (panel C in Fig. 1).
The number of CD3+CD4+ and CD3+CD8+ T cells were also determined. There was a strong influx of CD3+CD4+ T cells in M. vaccae-infected mice (panel A in Fig. 2), with the numbers reaching the highest levels at week 4 relative to the other groups (p < 0.01), then declining. However, the M. bovis BCG-infected group did not experience such a rapid decline. There was a rapid influx of CD8 cells, with the highest levels occurring on weeks 6 and 8 of M. vaccae infection compared with the other groups (p < 0.01; panel B in Fig. 2). These results were consistent with the histopathology analysis at week 2, in which we observed many more lymphocytic lesions in M. vaccae- infected mice (panel B in Fig. 1).
To better understand the Th1 response obtained during M. vaccae infection, we examined the number of CD3+ and CD4+ IFN-γ+ cells in the M. vaccae-infected mice. We found that the number of CD3+CD4+IFN-γ+ cells in the M. vaccae-infected mice increased during week 3 and decreased during week 6 (panel C in Fig. 2). Moreover, a strong Th1 response was associated with the emergence of CD3+CD4+IFN-γ+cells initially induced by M. vaccae. In contrast, M. bovis BCG induced a Th1 response that did not decline as quickly.
We also evaluated the Th2 response, which was characterized by the emergence of CD3+CD4+IL4+ cells. As shown in panel D in Fig. 2, a decrease in CD3+CD4+IL4+ cells in the M. vaccae-infected mice occurred during the third week, while the number of cells increased in the sixth week. M. bovis BCG-infected mice did not have an increased Th2 response.
We demonstrated that the serum from the M. vaccae-infected mice contained more IFN-γ starting in the first week of infection and that they had a higher level of IFN-γ in week 4 than the other groups (p < 0.001). Further analysis showed similar results for TNF-α and MCP-1, as well as the production of IL-12. Moreover, IL-4, IL-10 and TGF-β were higher in the M. vaccae-infected mice than in the M. bovis BCG group and the normal group, especially after week 4 (p < 0.001; Fig. 3).
The major producers of type I IFNs are DCs and plasmatoid DC cells [26]. As shown in Fig. 4A, we observed an increased number of DCs during M. vaccae infection to levels higher than were observed in response to M. bovis BCG infection from week 2 to week 4. Similarly, an increased number of pDCs was observed during M. vaccae infection from week 2 to week 8 to levels higher than occurred in response to M. bovis BCG infection (panel B in Fig. 4).
We evaluated the number of CD4+CD25+Foxp3+ regulatory T cells in the M. vaccae-infected mice. We found evidence of a large number of CD4+CD25+Foxp3+ regulatory T cells in the M. vaccae group (p < 0.001), as well as rapid expression of the cells at week 4 (p < 0.001). A smaller number of these cells was observed in the M. bovis BCG group (p < 0.05; panel A in Fig. 5).
To further investigate the expression of immunosuppressive Treg cells in the M. vaccae-infected mice, we examined mouse CD152 (CTLA-4). The expression level of CD152 can directly or indirectly regulate the level of Treg cells, and it plays a negative regulatory role in the immune response [26]. We found a stronger influx of CD4+CD25+CD152+ T cells during M. vaccae infection from week 1 to week 3 than in M. bovis BCG-infected and normal mice, but these cells declined after 4 weeks of M. vaccae infection in conjunction with a reduced number of CD3+CD4+ T cells (panel B in Fig. 5). In contrast, M. bovis BCG-infected mice contained a large number of CD4+CD25+CD152+ T cells after 4 weeks (p < 0.001). Therefore, the decreased expression of CD152 by M. vaccae-infected mice led to a lack of CD152 signaling.
Over the past few decades, researchers have exerted intense efforts to elucidate the host immune mechanisms activated in response to infection with NTM, but have had limited success [1729]. M. vaccae is a fast-growing species of saprophytic bacteria that is widely distributed [6]. It is interesting to note that most of the patients with pulmonary M. vaccae infection had a history of life on cattle farms [15]. However, further studies are needed to investigate the epidemiology, clinical manifestations, and host immune mechanisms of live M. vaccae infection because studies conducted to date have focused on heat-killed M. vaccae. It is known that heat-killed M. vaccae can cause decreased Th2 responses and increased Treg and Th1 responses [1936], which can protect the host against tuberculosis infection. However, to the best of our knowledge, it is currently unknown if these changes occur following infection with living M. vaccae.
In this research, we investigated the host immune responses against live M. vaccae infection. The results showed that the isolated M. vaccae was clearly more virulent in mice than M. bovis BCG, grew much faster in lungs and induced severe lung damage and consolidation, especially during the first 2 weeks of infection. After 2 weeks, the infection and lung pathology slowed due to the protective Th1 response induced by M. vaccae, including a high level of TNF-α and IFN-γ production. Furthermore, M. vaccae inhibited the Th2 response, including lowering the level of IL-4. Previous studies have shown that heat-killed M. vaccae can induce a Th1 response and inhibit the effects of Th2 response [39], which was similar to the results of the present study. However, the Th1 response subsequently declined in conjunction with the emergence of Treg cell response after week 4. There was also decreased production of IFN-γ and TNF-α and evidence of increased Th2 response, which was associated with a high level of IL-4 and differed from previous studies. There are several possible reasons for these differences. Specifically, we utilized live M. vaccae administered intravenously at 1 × 106 bacilli per animal. Additionally, we investigated the effects in healthy mice, whereas previous studies evaluated the immune-protective effects of heat-killed M. vaccae or M. tuberculosis in infected mice that were subjected to multiple immunizations with 107 bacilli via tail intravenous injection [3840]. Therefore, our model reflects those individuals infected with live M. vaccae more clearly.
Earlier studies of M. tuberculosis hypothesized that the type I IFNs were one type of protection for infected animals [18]. A high level of DCs and pDCs, which are type I IFN-rich sources [5], was observed in the M. vaccae-infected group. In the present study, DCs and pDCs showed a significant response to M. vaccae. We also evaluated the T-cell response during infection. CD3+CD4+IFN-γ+ cell numbers increased rapidly, but then declined at the fourth week after infection, whereas we observed a rapid increase in CD3+CD4+IL4+ cells and large production of IL-10 or TGF-β. Some immunosuppressive cytokines such as IL-10 and TGF-β have previously been confirmed to negatively influence the production of TNF and IFN-γ during mycobacterial infection [20]; therefore, we observed low inductions of TNF-α and IFN-γ from the fourth week, which were associated with the emergence of a high level IL-10 or TGF-β production. The function of Treg cells, which are characterized as CD4+CD25+FoxP3+ cells [28], during M. tuberculosis infections is not yet clear because their role has primarily been evaluated during early immune response to such infections [34]. Treg cells that delay T cell priming are present in infected tissue, and mycobacterial immune recovery of patients with HIV infection disease seems to lead to generation of a large number of Treg cells [30]. However, there has been little research on the function of regulatory T cells during M. vaccae infection. The results of the present study indicated that the presence of Treg cells was associated with inhibition of the acquired immune response by a reduction in IFN-γ in M. vaccae–infected mice. Furthermore, we determined the number of CD4+CD25+CD152+ cells in M. vaccae-infected mice, which was associated with the expression of immunosuppressive Treg cells and was down-regulated by week 4. We believe that the decreased expression of CD152 during M. vaccae-infection at week 4 and the coinciding decrease in IFN-γ levels represents a lack of CD152 and fewer activated T cells.
Our study describes the immune processes involved in host defense against infections with M. vaccae. Several different immune responses differed compared to heat-killed M. vaccae infection, and we demonstrated that the M. vaccae isolate was more virulent. Moreover, our study showed several new aspects of the immune response during NTMs infection, which may help improve our understand NTMs and provide a solid basis for treatment strategies.
Figures and Tables
Fig. 1
Lung histology of Mycobacterium (M.) vaccae and M. bovis Bacillus Calmette-Guérin (BCG)-infected mice. (A) Bacterial counts in the lungs at weeks 2, 4, 6 and 8 after infection of mice infected with M. vaccae or M. bovis BCG. Student's t-test vs. the M. bovis BCG group, **p < 0.01, ***p < 0.001. (B) Representative lung acid fast staining bacilli two weeks after M. vaccae or M. bovis BCG infection. (C) Representative lung histopathology in infected animals on weeks 2, 4, 6 and 8 of the infection. H&E stain. 800× (B), 40× (C).
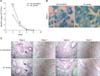
Fig. 2
Early infiltration of CD4+, CD8+ T cells, CD3+CD4+ IFN-γ+ cells and CD3+ CD4+ IL4+ cells in the lungs of mice infected with M. vaccae and M. bovis BCG. (A) Total number of cells expressing CD3+CD4+ surface markers. (B) CD3+CD8+ surface markers in the lungs of mice infected with various strains. (C) Number of CD3+ and CD4+ IFN-γ+ cells in M. vaccae-infected mice. (D) Number of CD3+ and CD4+ IL4+ cells in M. vaccae-infected mice. The results are expressed as the average ± SEM (n = 5 mice) of CD3+CD4+, CD3+CD8+ T cells, CD3+CD4+ IFN-γ+ cells or CD3+ and CD4+ IL4+ cells in the lungs. Student's t-test vs. the normal group, *p < 0.05, **p < 0.01, ***p < 0.001.

Fig. 3
Differential production of IFN-γ, TNF-α, MCP-1, IL-12p40, IL-10 and TGF-β cytokines in the serum of C57BL/6 mice infected with various bacterial strains (M. vaccae and M. bovis BCG). The levels of IFN-γ, TNF-α, MCP-1, IL-12p40, IL-10 and TGF-β were measured by ELISA. The results are expressed as the means ± SEM (n = 5 mice) in the serum. Student's t-test vs. the normal group, *p < 0.05, **p < 0.01, ***p < 0.001.

Fig. 4
Early infiltration of DCs and pDCs in the lungs of mice infected with the M. vaccae isolate and M. bovis BCG. Lung cells obtained from C57BL/6 mice infected with M. vaccae or M. bovis BCG and normal mice were assayed for the total number of pDCs (A) and DCs (B) using flow cytometry. The results are expressed as the average ± SEM of (n = 5 mice) pDCs or DCs in the lungs. Student's t-test vs. the normal group, *p < 0.05, **p < 0.01, ***p < 0.001.

Fig. 5
CD4+CD25+Foxp3+ cells in infected mice. Mice infected with M. vaccae showed a substantial increase in CD4+CD25+Foxp3+ regulatory T cells in the lungs relative to the M. bovis BCG and normal groups (A). Mice infected with M. vaccae showed a significant increase in CD4+CD25+CD152+T cells in the lungs relative to the M. bovis BCG and normal groups of mice at 3 weeks, whereas a larger number was observed in M. bovis BCG-infected mice beginning at 3 weeks (B). The results are expressed as the mean number ± SEM of CD4+CD25+Foxp3+ or CD4+CD25+CD152+ T cells (n = 5 mice) in the lungs. Student's t-test vs. the normal group, *p < 0.05, **p < 0.01, ***p < 0.001.

Acknowledgments
This work was supported by the National Natural Science Foundation of China (31272566, 31272541, 31272552 and 81170358), the Jilin Province Department of Education Science and Technology Program of China (2014270), and the Changchun Teacher University National Natural Science Foundation of China (201208).
References
1. Aksamit TR, Philley JV, Griffith DE. Nontuberculous mycobacterial (NTM) lung disease: the top ten essentials. Respir Med. 2014; 108:417–425.

2. Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Döffinger R, Bernaudin F, Jeppsson O, Gollob JA, Meinl E, Segal AW, Fischer A, Kumararatne D, Casanova JL. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998; 280:1432–1435.

3. Appelberg R, Castro AG, Pedrosa J, Silva RA, Orme IM, Minóprio P. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and -dependent phases of Mycobacterium avium infection. Infect Immun. 1994; 62:3962–3971.

4. Arlotta A, Cefalo MG, Maurizi P, Ruggiero A, Dodi I, Riccardi R. Critical pulmonary infection due to nontuberculous mycobacterium in pediatric leukemia: report of a difficult diagnosis and review of pediatric series. J Pediatr Hematol Oncol. 2014; 36:66–70.

5. Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med. 2005; 202:461–465.
6. Bönicke R, Juhasz SE. Description of new species Mycobacterium vaccae no. sp. Zentralbl Bakteriol Parasitenkd Infekt Hyg. 1964; 192:133–135.
7. Brown-Elliott BA, Wallace RJ Jr. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev. 2002; 15:716–746.

8. Daley CL, Griffith DE. Pulmonary disease caused by rapidly growing mycobacteria. Clin Chest Med. 2002; 23:623–632. vii

9. Dheda K, Booth H, Huggett JF, Johnson MA, Zumla A, Rook GAW. Lung remodeling in pulmonary tuberculosis. J Infect Dis. 2005; 192:1201–1209.

10. Dorman SE, Picard C, Lammas D, Heyne K, van Dissel JT, Baretto R, Rosenzweig SD, Newport M, Levin M, Roesler J, Kumararatne D, Casanova JL, Holland SM. Clinical features of dominant and recessive interferon gamma receptor 1 deficiencies. Lancet. 2004; 364:2113–2121.

11. Egelund EF, Fennelly KP, Peloquin CA. Medications and monitoring in nontuberculous mycobacteria infections. Clin Chest Med. 2015; 36:55–66.

12. Feng CG, Jankovic D, Kullberg M, Cheever A, Scanga CA, Hieny S, Caspar P, Yap GS, Sher A. Maintenance of pulmonary Th1 effector function in chronic tuberculosis requires persistent IL-12 production. J Immunol. 2005; 174:4185–4192.

14. Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015; 33:563–577.
15. Hambleton S, Salem S, Bustamante J, Bigley V, Boisson-Dupuis S, Azevedo J, Fortin A, Haniffa M, Ceron-Gutierrez L, Bacon CM, Menon G, Trouillet C, McDonald D, Carey P, Ginhoux F, Alsina L, Zumwalt TJ, Kong XF, Kumararatne D, Butler K, Hubeau M, Feinberg J, Al-Muhsen S, Cant A, Abel L, Chaussabel D, Doffinger R, Talesnik E, Grumach A, Duarte A, Abarca K, Moraes-Vasconcelos D, Burk D, Berghuis A, Geissmann F, Collin M, Casanova JL, Gros P. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med. 2011; 365:127–138.

16. Haug M, Awuh JA, Steigedal M, Frengen Kojen J, Marstad A, Nordrum IS, Halaas Ø, Flo TH. Dynamics of immune effector mechanisms during infection with Mycobacterium avium in C57BL/6 mice. Immunology. 2013; 140:232–243.

17. Henkle E, Winthrop KL. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin Chest Med. 2015; 36:91–99.

18. Hernandez-Pando R, Pavon L, Orozco EH, Rangel J, Rook GA. Interactions between hormone-mediated and vaccine-mediated immunotherapy for pulmonary tuberculosis in BALB/c mice. Immunology. 2000; 100:391–398.

19. Jönsson B, Ridell M, Wold AE. Non-tuberculous mycobacteria and their surface lipids efficiently induced IL-17 production in human T cells. Microbes Infect. 2012; 14:1186–1195.

20. Kobayashi T, Morino E, Takasaki J, Nagahara Y, Sugiyama H. Nontuberculous mycobacterial osteomyelitis in human immunodeficiency virus-negative patients: a case series. Jpn J Infect Dis. 2016; 69:149–150.

21. Koehne G, Maddux R, Britt J. Rapidly growing mycobacteria associated with bovine mastitis. Am J Vet Res. 1981; 42:1238–1239.
22. Lienhardt C, Azzurri A, Amedei A, Fielding K, Sillah J, Sow OY, Bah B, Benagiano M, Diallo A, Manetti R, Manneh K, Gustafson P, Bennett S, D'Elios MM, McAdam K, Del Prete G. Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo. Eur J Immunol. 2002; 32:1605–1613.

23. Monack DM, Mueller A, Falkow S. Persistent bacterial infections: the interface of the pathogen and the host immune system. Nat Rev Microbiol. 2004; 2:747–765.

24. Ordway D, Henao-Tamayo M, Harton M, Palanisamy G, Troudt J, Shanley C, Basaraba RJ, Orme IM. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent Th1 response followed by rapid down-regulation. J Immunol. 2007; 179:522–531.

25. Ottenhoff TH, Verreck FAW, Hoeve MA, van de Vosse E. Control of human host immunity to mycobacteria. Tuberculosis (Edinb). 2005; 85:53–64.

26. Quinn KM, McHugh RS, Rich FJ, Goldsack LM, de Lisle GW, Buddle BM, Delahunt B, Kirman JR. Inactivation of CD4+ CD25+ regulatory T cells during early mycobacterial infection increases cytokine production but does not affect pathogen load. Immunol Cell Biol. 2006; 84:467–474.

27. Ramsdell F. Foxp3 and natural regulatory T cells: key to a cell lineage? Immunity. 2003; 19:165–168.
28. Hachem R, Raad I, Rolston KVI, Whimbey E, Katz R, Tarrand J, Libshitz H. Cutaneous and pulmonary infections caused by Mycobacterium vaccae. Clin Infect Dis. 1996; 23:173–175.
29. Rottman M, Catherinot E, Hochedez P, Emile JF, Casanova JL, Gaillard JL, Soudais C. Importance of T cells, gamma interferon, and tumor necrosis factor in immune control of the rapid grower Mycobacterium abscessus in C57BL/6 mice. Infect Immun. 2007; 75:5898–5907.

30. Seddiki N, Sasson SC, Santner-Nanan B, Munier M, van Bockel D, Ip S, Marriott D, Pett S, Nanan R, Cooper DA, Zaunders JJ, Kelleher AD. Proliferation of weakly suppressive regulatory CD4+ T cells is associated with over-active CD4+ T-cell responses in HIV-positive patients with mycobacterial immune restoration disease. Eur J Immunol. 2009; 39:391–403.

31. Shang S, Gibbs S, Henao-Tamayo M, Shanley CA, McDonnell G, Duarte RS, Ordway DJ, Jackson M. Increased virulence of an epidemic strain of Mycobacterium massiliense in mice. PLoS One. 2011; 6:e24726.
32. Shimizu K, Hirose T, Sato M, Tsukamura M. Isolation of acid-fast organisms resembling Mycobacterium vaccae from a lesion of bovine nodular thelitis. Microbiol Immunol. 1977; 21:469–472.

33. Starke JR. Committee on Infectious Diseases. Interferon-γ release assays for diagnosis of tuberculosis infection and disease in children. Pediatrics. 2014; 134:e1763–e1773.
34. Urdahl KB, Shafiani S, Ernst JD. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol. 2011; 4:288–293.

35. Velayati AA, Farnia P, Mozafari M, Mirsaeidi M. Nontuberculous mycobacteria isolation from clinical and environmental samples in Iran: twenty years of surveillance. Biomed Res Int. 2015; 2015:254285.

36. von Reyn CF, Mtei L, Arbeit RD, Waddell R, Cole B, Mackenzie T, Matee M, Bakari M, Tvaroha S, Adams LV, Horsburgh CR, Pallangyo K. DarDar Study Group. Prevention of tuberculosis in Bacille Calmette-Guerin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. AIDS. 2010; 24:675–685.

37. Wang C, Qi H, Jiang X, Chen FF, Ma H, Wang C, Qian A. Molecular differentiation of nontuberculous Mycobacterium isolated from different animals. Appl Mech Mater. 2013; 421:300–303.

38. Wang CC, Rook GA. Inhibition of an established allergic response to ovalbumin in BALB/c mice by killed Mycobacterium vaccae. Immunology. 1998; 93:307–313.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download