Abstract
Curcumin protects the skin against radiation-induced epidermal damage and prevents morphological changes induced by irradiation skin, thereby maintaining the epidermal thickness and cell density of basal layers. In this study, the effects of topical curcumin treatment on radiation burns were evaluated in a mini-pig model. Histological and clinical changes were observed five weeks after radiation exposure to the back (60Co gamma-radiation, 50 Gy). Curcumin was applied topically to irradiated skin (200 mg/cm2) twice a day for 35 days. Curcumin application decreased the epithelial desquamation after irradiation. Additionally, when compared to the vehicle-treated group, the curcumin-treated group showed reduced expression of cyclooxygenase-2 and nuclear factor-kappaB. Furthermore, irradiation prolonged healing of biopsy wounds in the exposed area, whereas curcumin treatment stimulated wound healing. These results suggest that curcumin can improve epithelial cell survival and recovery in the skin and therefore be used to treat radiation burns.
Skin burns are not only a common side effect of therapeutic irradiation, but are also the most common injury in radiation-related accidents [1011]. While numerous tissues and organs are affected by radiation exposure in radiotherapy, the skin, which covers the entire body, is the site of immediate and severe damage [6]. Moreover, the side effects of irradiation in a growing number of cancer survivors and the need for medical countermeasures against radiologic or nuclear accidents and terrorism have sparked interest in determining novel ways to ameliorate radiation-induced tissue damage [8]. Numerous promising compounds have been investigated for their protective effects against radiation-induced injuries [14171933]. The side effects of irradiation can reduce the quality of life and be dose-limiting, resulting in reduced treatment for the patient. Therefore, it is necessary to develop drugs that can treat these side effects with low toxicity and at a relatively low price. A number of studies have evaluated the radioprotective potential of natural products, including various plants and herbs, with the hope that their active components and knowledge of their mechanisms of action will lead to the discovery of suitable pharmacological agents that could protect humans against the deleterious effects of ionizing radiation [142133]. Although most of these studies have focused on protective effects, therapeutic effects of natural products at the injury site would make the potential treatment agent more suitable for application in a mass casualty situation or an unforeseen nuclear accident.
Curcumin, a constituent of Curcuma longa (family Zingiberaceae) and an important active component of turmeric, has been shown to promote healing of skin wounds in various models [3132], including radiation-induced skin wounds [152429]. Furthermore, curcumin was found to be a potent antioxidant and effective radioprotective agent [11327].
Curcumin was previously shown to protect against radiation-induced dermatitis in a mouse model. Although small animals such as mice have frequently been used to investigate the radioprotective effect of curcumin [1524], experiments using small animals have limited translational value because of the anatomical and pathophysiological differences between animal and human tissues. However, the anatomical and physiological similarities between humans and pigs make the pig an optimal animal model for evaluation of human skin damage and wound healing [1835]. Therefore, in this study, we examined the effects of topical application of curcumin on the skin after gamma-radiation exposure using a mini-pig model.
Male Göttingen mini-pigs (mean weight, 19 kg; range, 18–20 kg; age, 6–7 months) obtained from PWG Genetics (Korea) were used in these experiments. The mini-pigs were provided with tap water and commercial laboratory piglet chow from Purina Korea (Purina laboratory pig chow-38075) containing crude protein, fat, fiber, and ash, as well as calcium, phosphorus, and moisture (14.5, 4, 5, 8, 0.55, 1, and 14%, respectively). In addition, no antibiotic supplements were used. All animal experiments were performed according to the protocol approved by the Institutional Animal Care and Use Committee of the Korea Institute of Radiological and Medical Sciences (KIRAMS).
To prepare a formulation of curcumin appropriate for topical administration, 200 mg of carbopol (Carbopol 934P; Lubrizol, USA) was added to 2.5 mL of distilled water and 200 mg of curcumin was solubilized in 2 mL of ethanol. The ethanolic dispersion of curcumin and an appropriate amount of ethanol were transferred to an aqueous dispersion of carbopol. Methanol (1.25 mL) was mixed with 1 mL of ethanol and added to the curcumin and carbopol mixture, which was gradually stirred, and carbopol was allowed to soak for 2 h. Triethanolamine (100 mg; Sigma-Aldrich, USA) was added to neutralize the carbopol solution and facilitate the formation of a gel, after which the pH was adjusted to 6.8. The vehicle cream was prepared using the same ingredients and identical methods as the curcumin cream, but curcumin was omitted from the mixture. This topical gel formulation was previously shown to result in the highest permeability of curcumin without causing skin irritation or anti-inflammatory effects [26]. For topical treatment, curcumin or the vehicle cream (concentration, 200 mg/cm2) was spread on the irradiated skin of pigs twice daily for 35 days, and the first application was performed immediately after irradiation.
To observe the effects of gamma-radiation on the skin of mini-pigs (3 animals per group), the dorsal skin was irradiated. For all procedures, animals were anesthetized with tiletamine/zolazepam (Zoletil 50; Virbac Korea, Korea) and medetomidine (Domitol; Pfizer Animal Health Korea, Korea). Three to four days prior to irradiation, the fur of the animals was clipped from areas that were to be exposed, and the positions of the exposure fields were marked and tattooed using India ink. The fields were gamma-irradiated at a dose of 50 Gy using 60Co gamma-rays (Theratron 780; AECL, Canada) at a dose rate of 130.1 cGy/min (field size, 5 × 2 cm, rectangular; source-to-skin distance, 80 cm; depth, 1 cm with bolus 1 cm). Based on the area of the flank skin available, 50 Gy irradiation was administered to each pig (Fig. 1).
The pigs were carefully evaluated every week during the five weeks following irradiation, and their skin reactions were scored using a clinical status scoring system. The presence and appearance of skin reactions and the characteristics of the operation scar were examined. The following scoring system was used to measure the reactions based on previous skin damage models [38]: grade 1.0, normal skin; grade 1.5, minimal erythema and slightly dry skin; grade 2.0, moderate erythema and dry skin; grade 2.5, marked erythema and dry desquamation; grade 3.0, dry desquamation and minimal dry crusting; grade 3.5, dry desquamation, dry crusting, and minimal superficial scabbing; grade 4.0, patchy moist desquamation and moderate scabbing; grade 4.5, confluent moist desquamation, ulcers, and large deep scabs; grade 5.0, open wound and full-thickness skin loss; and grade 5.5, necrosis [1838].
A 5 mm punch biopsy was performed under anesthesia to obtain a skin sample from the non-irradiated healthy skin and the irradiated skin area 3, 7, 21, and 35 days after irradiation. After collection, skin biopsy samples were pinned to a cork to maintain the 5 mm size. Biopsy samples of non-irradiated skin were obtained from each pig before irradiation. All biopsy samples were processed and embedded in paraffin wax after fixation in 10% buffered formalin, then cut into 4 µm thick coronal sections and deparaffinized. Next, the sections were stained with hematoxylin and eosin and examined by optical microscopy. The longest rete ridge on each slide was selected and measured from the bottom of the basal layer to the bottom of the stratum corneum, avoiding the areas in which the inclusion appeared to be oblique. The mean values were then calculated based on the measurements of each section on each slide. The cell density of the basal layers was determined by counting the cells in the basement membrane at a depth of at least 5 mm. The results were expressed as the number of cells per millimeter of basement membrane. Degenerated cells (i.e., cells exhibiting pyknosis and shrinkage necrosis) were excluded from these calculations [18].
After incubation in normal horse serum for 60 min to prevent nonspecific binding, the skin sections were incubated with mouse anti-nuclear factor (NF)-κB (sc-109, 1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and mouse anti-COX-2 (18-7379, 1:200; Zymed, USA) in phosphate buffered saline-Tween (PBS-T overnight at 4℃. The sections were subsequently incubated with biotinylated horse anti-mouse IgG (VECTASTAIN Elite ABC Kit; Vector Laboratories, USA). The immunoreactivity was assessed using the avidin–biotin peroxidase complex (VECTASTAIN Elite ABC Kit; Vector Laboratories). The peroxidase reaction was developed using a diaminobenzidine substrate kit (DAB Substrate Kit SK-4100; Vector Laboratories). As a control, the primary antibodies were omitted from the immunohistochemical analysis of a few test sections in each experiment. The sections were then counterstained with hematoxylin before being mounted.
Blood samples were collected via the ear vein into sample tubes containing ethylenediaminetetraacetic acid at different time points (before irradiation, 3, 7, 21, and 35 days after irradiation). Peripheral eosinophils were automatically counted using a Hemavet System (Drew Scientific, UK).
The data were expressed as the means ± the standard error of the mean (SEM) values. Differences between groups were evaluated by one-way analysis of variance (ANOVA) followed by a Student-Newman-Keuls post hoc test for multiple comparisons. In all cases, a p < 0.05 was considered significant.
Time-dependent gross changes in the irradiated skin were observed in both vehicle- and curcumin-treated mini-pigs 35 days after radiation exposure. One week after irradiation, the exposed area of the skin showed desquamation associated with bright-red erythema (panel B in Fig. 1). This reaction increased progressively in severity over the first 5 weeks following irradiation, with persistent moist desquamation and tissue breakdown that progressed to the dermis (Fig. 1). In both the vehicle- and curcumin-treated mini-pigs, the clinical changes were similar when assessed 1 week after irradiation. However, the beneficial effects of curcumin treatment on the dermatitis appeared 2 weeks after irradiation. The curcumin-treated group exhibited a decreased severity in the skin reaction compared to that of the vehicle-treated irradiation group (Fig. 2).
Hematoxylin and eosin stained sections were examined to assess the basal cell density and epithelial depth in pig skin with or without curcumin treatment. Skin sections that were collected from each pig before irradiation exhibited normal morphology. The basal cell density and epithelial layer thickness changed in parallel with the observed progression of clinical alterations (Fig. 3).
Radiation exposure of the skin gradually decreased the density of basal cells in the epidermis until 5 weeks after irradiation. However, the decreased basal cell counts were significantly ameliorated 21 and 35 days after irradiation (p < 0.01 and p < 0.05 vs. vehicle-treated irradiation group, respectively; Fig. 3).
The thickness of the epidermis markedly decreased gradually five weeks after irradiation. However, curcumin treatment prevented this decrease 35 days after irradiation (p < 0.01 vs. vehicle-treated irradiation group), likely by preserving the basal cell numbers. These results suggest that curcumin significantly alleviates skin injury in irradiated pig skin (Fig. 3).
In normal pigskin, the cyclooxygenase-2 (COX-2) staining was minimal with some staining in the sebaceous glands and subcutis, but no visible epidermal staining (panel A in Fig. 4). COX-2 expression was detectable in the epidermis of the irradiated skin, and evaluation between 1 and 3 weeks following exposure revealed COX-2 expression in the granular layer and the stratum corneum (panels B and C in Fig. 4). Five weeks after irradiation, patchy areas of staining were observed in all skin layers (panel D in Fig. 4). However, COX-2 expression in the irradiated skin was lower in the curcumin-treated skin than in the vehicle-treated skin (panels E-G in Fig. 4).
In normal pigskin, NF-κB staining was minimal, with some staining evident in the sebaceous glands, hair follicles, and epidermis. Additionally, NF-κB was expressed in the cytoplasmic region of the basal level of the epidermis, while no nuclear staining was detected (panel A in Fig. 5). NF-κB expression in irradiated skin increased between 1 and 2 weeks following irradiation (panels B and C in Fig. 5). Moreover, diffuse cytoplasmic staining was observed in all epidermal layers three weeks after irradiation, while nuclear staining was detected after five weeks (panel D in Fig. 5). In the curcumin-treated irradiated skin, NF-κB expression was lower than it was in the vehicle-treated irradiated skin, and the nuclear expression rapidly decreased (panels E-G in Fig. 5).
Focal radiation exposure transiently decreased the white blood cell count of the peripheral blood, including neutrophils and lymphocytes, 7 days after exposure. Analysis of the peripheral blood sample of the curcumin-treated group did not show a radioprotective effect of curcumin initially. However, 21 and 35 days after irradiation, focal radiation-induced skin inflammation increased the neutrophil count of the peripheral blood. Conversely, the curcumin-treated group showed decreased neutrophils and eosinophils in the blood, although the decrease was not significant, suggesting that curcumin attenuated radiation-induced skin inflammation (Fig. 6).
Healing of the biopsy wound progressed with time, with steady healing of the skin wound observed before irradiation. The biopsy wound healed within 6 to 12 days in normal skin, but the biopsy lesions did not appear to be healing 3 days after irradiation. Exposure of skin to irradiation significantly delayed wound healing, as observed at various post-irradiation time points. However, curcumin treatment attenuated the radiation-induced delayed healing of the biopsy wound 3 days after irradiation (Fig. 7).
This study was conducted to assess the therapeutic properties of curcumin in an irradiated pigskin model by examining the level of damage to skin cells and the progression of wound healing in skin tissue subjected to irradiation. Curcumin ameliorated skin injury and promoted cell survival in pigskin. Deleterious effects of ionizing radiation on the skin are secondary to the production of free radicals and release of inflammatory mediators and cytokines [22]. Therefore, previous experimental and clinical studies conducted using drugs with known anti-inflammatory, antioxidant, and cytoprotective properties showed that they reduced radiation-induced skin injury [62022]. Numerous previous studies have demonstrated the topical antioxidant and anti-inflammatory effects of curcumin at the site of administration [16]. Curcumin is known to have several biochemical properties, including maintenance of membrane structure and function, as well as to play important roles in skin and connective tissue metabolism and wound healing [162532]. Furthermore, curcumin contributes to the maintenance of epithelial and tissue integrity by promoting cell growth and suppressing apoptosis, in addition to its under-appreciated role as an antioxidant that protects against free radical damage during inflammatory responses [162532]. Topical use of curcumin has been reported to stimulate skin wound healing by enhancing re-epithelialization, decreasing reactive oxygen species and inflammation, and modulating collagen [25]. Curcumin has been shown to protect against radiation-induced skin dermatitis in a mouse model [1524].
Ionizing radiation is a direct and indirect activator of NF-κB, which up-regulates the transcription of COX-2, a stress response protein [1839]. In this study, an increase in NF-κB and COX-2 expression was observed in epidermal cells following irradiation. Although it has recently been suggested that NF-κB plays a protective role in radiation-induced intestinal tissue changes [7], other studies have reported that NF-κB increases the severity of the injury and inflammation at sites upstream of COX-2 [1834]. Moreover, COX-2 is known to stimulate the synthesis of prostaglandins involved in the inflammatory cascade, which culminates in tissue injury [39]. Curcumin modulates tissue inflammatory response by downregulating the activity of COX-2 and NF-κB [9]. COX-2 inhibition is likely mediated via the curcumin-induced suppression of NF-κB activation [36]. Curcumin is believed to suppress NF-κB activation and proinflammatory gene expression by blocking phosphorylation of the inhibitory factor I-κB kinase. The suppression of NF-κB activation downregulates the expression of COX-2 expression, which inhibits the inflammatory process [36]. In an animal model of inflammation, curcumin was shown to inhibit arachidonic acid metabolism and suppress inflammation in the mouse epidermis by downregulating the COX and lipoxygenase pathways [12]. The results of the current study demonstrate that curcumin inhibits irradiation-induced increases in COX-2 and NF-κB expression in skin epithelial cells. Decreased COX-2 and NF-κB expression were found to occur in parallel with the attenuation of clinical and histological skin tissue changes following irradiation. In a previous study, celecoxib, a COX-2 inhibitor, reduced skin damage after irradiation and decreased the infiltration of neutrophils in locally irradiated skin tissue. The effects of celecoxib on inflammation help explain its protective effects in irradiated cutaneous tissues [23].
Radiation can impair the healing process, resulting in inflammation, thinning of the granulation tissue, and delayed re-epithelialization [5]. Wound healing of irradiated lesions was delayed, indicating that radiation exposure alters the local conditions of the wound and adversely affects wound repair. A previous study demonstrated that curcumin pretreatment could be used as a protective therapy to ameliorate radiation-induced delay in wound repair in the case of radiation-induced skin injuries [15]. In this study, biopsy wounds within the irradiated lesions did not heal following treatment with vehicle, and the severity of the local tissue reaction to injury progressed with time. However, the application of curcumin gradually ameliorated the biopsy wound, improving its clinical appearance compared with that of the vehicle-treated group.
Previous studies demonstrated that oral administration of curcumin protected against radiation-induced skin dermatitis in patients with breast cancer [1524]. However, topical application has advantages including that the treatment remains localized to the affected area. When curcumin is used as an oral medication, it might be transported through the bloodstream, affecting the entire body and not just the damaged area. Curcumin is poorly absorbed following oral or intraperitoneal administration [28], and only trace amounts of the compound are detected in the blood. Curcumin undergoes extensive first-pass metabolism [4] and is therefore a suitable candidate for topical gel formulations. The topical route of application holds great promise as an effective and safe method of administering curcumin to treat skin injuries [26]. Because most inflammatory diseases develop locally and near the surface of the body, topical application of curcumin to the site of inflammation offers the advantage of delivering the drug directly to the site of disease and thereby producing local effects [23]. However, the barrier properties of intact skin limit the permeability of a wide variety of substances including pharmaceutically active agents [30]. A recent study established the suitability of the transdermal route of drug delivery for the administration of curcumin [26]. Additionally, this formulation treatment was found to elicit anti-inflammatory effects against carrageenan-induced rat paw edema and skin irritation [26]. In this study, we investigated the benefits of topical application of curcumin against radiation-induced skin damage in a piglet model.
The topical gel formulation was previously shown to result in the highest permeability of curcumin without causing skin irritation and anti-inflammatory effects [26]. A previous study showed the cumulative amount of the drug that permeated through the rat epidermis from a topical gel formulation [26]. The amount of curcumin that permeated during the 24 h study was 1212.08 ± 32.44 µg cm−2. The flux was obtained by dividing the cumulative amount of drug permeated per cm2 of the skin with time. Therefore, the corresponding flux of curcumin was 46.85 ± 1.76 µg cm−2 h−1 [26].
Focal radiation exposure transiently decreased the white blood cell count, including neutrophils and lymphocytes, in the peripheral blood. Analysis of the peripheral blood of the curcumin-treated group did not show a radioprotective effect of curcumin initially. This finding suggests that topical treatment of curcumin may not affect the entire body. However, focal irradiation induced skin inflammation by increasing neutrophils in the peripheral blood. The curcumin-treated group showed decreased neutrophils and eosinophils in the blood, although the decrease was not significant, suggesting that curcumin attenuated radiation-induced skin inflammation. The protective effects of curcumin against radiation burns were investigated using a rodent model and the ability of curcumin to decrease the severity and duration of inflammation were assessed in irradiated mice [1524]. However, the murine model has severe limitations, including the anatomical and pathophysiological differences between mouse and human tissues. The physiology, biochemistry, and anatomy of pigs are phylogenetically close enough to those of humans to simulate human skin tissue damage. In addition, human and pig skin tissues have microscopically heterogeneous structures with similar morphology, cellular composition, and physiological properties [3537]. Pig tissue has been used to evaluate drug delivery, wound healing following heat or chemical burns, and as a model to evaluate light distribution in the skin [37]. Furthermore, use of the mini-pig enabled us to successfully perform sequential analyses on skin tissues from the same animals, which allowed examination of the sequential clinicopathologic changes and evaluation of the therapeutic effects of curcumin in radiation-induced skin damage.
In conclusion, curcumin exhibited beneficial effects in radiation burns by postponing the onset and decreasing the severity of radiation-induced dermatitis in a piglet model. When compared to the vehicle-treated group, the curcumin-treated group showed reduced expression of COX-2 and NF-κB in irradiated skin. Irradiation prolonged healing of biopsy wounds in the exposed area, but curcumin treatment stimulated wound-healing. These results provide the basis for further studies to evaluate the potential of curcumin for treating radiation-induced skin damage. Furthermore, it would be worthwhile to study the ability of curcumin applied during radiation treatment of patients with cancer to reduce radiation-induced toxicity.
Figures and Tables
 | Fig. 1(A) Focal gamma irradiation (50 Gy) applied to dorsal skin of mini-pigs. (B) Image of irradiated lesion and punch biopsy site. |
 | Fig. 2Curcumin attenuated clinical skin changes after irradiation (50 Gy). (A) Skin appearance before irradiation, 7, 21, and 35 days after irradiation of pigs treated with vehicle or curcumin. (B) Time-dependent changes in clinical score in vehicle- or curcumin-treated skin of pigs following irradiation. Data are the means ± the standard error of the mean (SEM). |
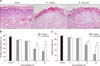 | Fig. 3Curcumin attenuated histological changes, including decreased basal cell numbers and skin depth after irradiation. (A) Histological skin changes 35 days after irradiation (50 Gy) of pigs treated with vehicle or curcumin. H&E stain. 400×. (B) Time-dependent changes in basal cell density in the skin of mini-pigs treated with vehicle or curcumin following irradiation. (C) Time-dependent changes in epidermal thickness of the skin of mini-pigs following irradiation and vehicle or curcumin treatment. Data are the means ± the standard error of the mean (SEM). *p < 0.05 and **p < 0.01 vs. vehicle-treated irradiated animals. |
 | Fig. 4Curcumin decreased expression of cyclooxygenase (COX)-2 in skin after irradiation (50 Gy). (A) COX-2 expression in skin before irradiation exposure. COX-2 expression in skin of vehicle-treated (B-D) or curcumin-treated (E-G) mini-pigs 7, 21, and 35 days after irradiation. Hematoxylin counterstain. 400× (A-G). |
 | Fig. 5Curcumin decreased the expression of nuclear factor (NF)-κB in skin after irradiation (50 Gy). (A) NF-κB expression in skin before irradiation exposure. NF-κB expression in skin of vehicle-treated (B-D) or curcumin-treated (E-G) mini-pigs 7, 21, and 35 days after irradiation. Hematoxylin counterstain. 400× (A-G). |
 | Fig. 6Peripheral blood counts before, 3, 7, 21, and 35 days after focal irradiation (50 Gy) and vehicle or curcumin treatment. Effect of curcumin treatment on (A) population of blood cells, (B) neutrophils, (C) eosinophils, (D) lymphocytes, (E) red blood cells, and (F) platelets. Data are the means ± SEM. |
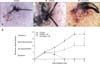 | Fig. 7Curcumin stimulated wound healing in biopsy lesions three days after irradiation (50 Gy). (A) Appearance of biopsy lesions of non-irradiated lesions 32 days after biopsy. Representative images showing biopsy lesions 35 days after irradiation and vehicle (B) or curcumin (C) treatment. (D) Time-dependent changes in biopsy wounds in skin of vehicle- or curcumin-treated pigs following irradiation. Curcumin administration affected biopsy wound formation in skin. Data are the means ± SEM. |
Acknowledgments
This study was supported by the Development of Therapeutic Improvement on Acute Radiation Syndrome (50581-2014) Project of the Ministry of Science, ICT and Future Planning, Korea.
References
1. Abraham SK, Sarma L, Kesavan PC. Protective effects of chlorogenic acid, curcumin and β-carotene against γ-radiation-induced in vivo chromosomal damage. Mutat Res. 1993; 303:109–112.

2. Arellano A, Santoyo S, Martin C, Ygartua P. Influence of propylene glycol and isopropyl myristate on the in vitro percutaneous penetration of diclofenac sodium from carbopol gels. Eur J Pharm Sci. 1999; 7:129–135.

3. Arellano A, Santoyo S, Martn C, Ygartua P. Surfactant effects on the in vitro percutaneous absorption of diclofenac sodium. Eur J Drug Metab Pharmacokinet. 1998; 23:307–112.

4. Asai A, Miyazawa T. Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci. 2000; 67:2785–2793.

5. Bernatchez SF, Parks PJ, Grussing DM, Matalas SL, Nelson GS. Histological characterization of a delayed wound healing model in pig. Wound Repair Regen. 1998; 6:223–233.

6. Bernstein EF, Sullivan FJ, Mitchell JB, Salomon GD, Glatstein E. Biology of chronic radiation effect on tissues and wound healing. Clin Plast Surg. 1993; 20:435–453.

7. Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, Kurnasov OV, Fort FL, Osterman AL, DiDonato JA, Feinstein E, Gudkov AV. An agonist of Toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008; 320:226–230.

8. Denham JW, Hauer-Jensen M, Peters LJ. Is it time for a new formalism to categorize normal tissue radiation injury? Int J Radiat Oncol Biol Phys. 2001; 50:1105–1106.

9. Goel A, Jhurani S, Aggarwal BB. Multi-targeted therapy by curcumin: how spicy is it? Mol Nutr Food Res. 2008; 52:1010–1030.

10. Hoashi T, Okochi H, Kadono T, Tamaki K, Nishida M, Futami S, Maekawa K. A case of acute radiation syndrome from the dermatological aspect. Br J Dermatol. 2008; 158:597–602.

11. Hopewell JW. The skin: its structure and response to ionizing radiation. Int J Radiat Biol. 1990; 57:751–773.

12. Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, Conney AH. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res. 1991; 51:813–819.
13. Inano H, Onoda M. Radioprotective action of curcumin extracted from Curcuma longa LINN: inhibitory effect on formation of urinary 8-hydroxy-2′-deoxyguanosine, tumorigenesis, but not mortality, induced by γ-ray irradiation. Int J Radiat Oncol Biol Phys. 2002; 53:735–743.

14. Jagetia GC. Radioprotective potential of plants and herbs against the effects of ionizing radiation. J Clin Biochem Nutr. 2007; 40:74–81.

15. Jagetia GC, Rajanikant GK. Curcumin treatment enhances the repair and regeneration of wounds in mice exposed to hemibody γ-irradiation. Plast Reconstr Surg. 2005; 115:515–528.

16. Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009; 14:141–153.
17. Kim JS, Yang M, Kim SH, Shin T, Moon C. Neurobiological toxicity of radiation in hippocampal cells. Histol Histopathol. 2013; 28:301–310.
18. Kim JS, Rhim KJ, Jang WS, Lee SJ, Son Y, Lee SS, Park S, Lim SM. β-irradiation (166Ho patch)-induced skin injury in the mini-pig: effects on NF-κB and COX-2 expression in the skin. J Vet Sci. 2015; 16:1–9.

19. Kim JS, Yang M, Lee CG, Kim SD, Kim JK, Yang K. In vitro and in vivo protective effects of granulocyte colony-stimulating factor against radiation-induced intestinal injury. Arch Pharm Res. 2013; 36:1252–1261.

20. Kim SH, Kim SR, Lee HJ, Oh H, Ryu SY, Lee YS, Kim TH, Jo SK. Apoptosis in growing hair follicles following gamma-irradiation and application for the evaluation of radioprotective agents. In Vivo. 2003; 17:211–214.
21. Kim SH, Lee HJ, Kim JS, Moon C, Kim JC, Park HR, Jung U, Jang JS, Jo SK. Protective effect of an herbal preparation (HemoHIM) on radiation-induced intestinal injury in mice. J Med Food. 2009; 12:1353–1358.

22. Kouvaris J, Kouloulias V, Kokakis J, Matsopoulos G, Myrsini B, Vlahos L. The cytoprotective effect of amifostine in acute radiation dermatitis: a retrospective analysis. Eur J Dermatol. 2002; 12:458–462.
23. Liang L, Hu D, Liu W, Williams JP, Okunieff P, Ding I. Celecoxib reduces skin damage after radiation: selective reduction of chemokine and receptor mRNA expression in irradiated skin but not in irradiated mammary tumor. Am J Clin Oncol. 2003; 26:S114–S121.
24. Okunieff P, Xu J, Hu D, Liu W, Zhang L, Morrow G, Pentland A, Ryan JL, Ding I. Curcumin protects against radiation-induced acute and chronic cutaneous toxicity in mice and decreases mRNA expression of inflammatory and fibrogenic cytokines. Int J Radiat Oncol Biol Phys. 2006; 65:890–898.

25. Panchatcharam M, Miriyala S, Gayathri VS, Suguna L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem. 2006; 290:87–96.

26. Patel NA, Patel NJ, Patel RP. Formulation and evaluation of curcumin gel for topical application. Pharm Dev Technol. 2009; 14:80–89.

27. Phan TT, See P, Lee ST, Chan SY. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J Trauma. 2001; 51:927–931.

28. Ravindranath V, Chandrasekhara N. Absorption and tissue distribution of curcumin in rats. Toxicology. 1980; 16:259–265.

29. Ryan JL, Heckler CE, Ling M, Katz A, Williams JP, Pentland AP, Morrow GR. Curcumin for radiation dermatitis: a randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat Res. 2013; 180:34–43.

30. Shah VP, Behl CR, Flynn GL, Higuchi WI, Schaefer H. Principles and criteria in the development and optimization of topical therapeutic products. J Pharm Sci. 1992; 81:1051–1054.

31. Sidhu GS, Mani H, Gaddipati JP, Singh AK, Seth P, Banaudha KK, Patnaik GK, Maheshwari RK. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair Regen. 1999; 7:362–374.

32. Sidhu GS, Singh AK, Thaloor D, Banaudha KK, Patnaik GK, Srimal RC, Maheshwari RK. Enhancement of wound healing by curcumin in animals. Wound Repair Regen. 1998; 6:167–177.

33. Son TG, Gong EJ, Bae MJ, Kim SD, Heo K, Moon C, Yang K, Kim JS. Protective effect of genistein on radiation-induced intestinal injury in tumor bearing mice. BMC Complement Altern Med. 2013; 13:103.

34. Sonis ST, O'Donnell KE, Popat R, Bragdon C, Phelan S, Cocks D, Epstein JB. The relationship between mucosal cyclooxygenase-2 (COX-2) expression and experimental radiation-induced mucositis. Oral Oncol. 2004; 40:170–176.

35. Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001; 9:66–76.

36. Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat Res. 2001; 480-481:243–268.

37. Swindle MM, Makin A, Herron AJ, Clubb FJ Jr, Frazier KS. Swine as models in biomedical research and toxicology testing. Vet Pathol. 2012; 49:344–356.

38. Wang XJ, Lin S, Kang HF, Dai ZJ, Bai MH, Ma XL, Ma XB, Liu MJ, Liu XX, Wang BF. The effect of Rhizoma Coptidis and Coptis chinensis aqueous extract on radiation-induced skin injury in a rat model. BMC Complement Altern Med. 2013; 13:105.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download