Abstract
Mycobacterium (M.) bovis causes tuberculosis and has a broad host range, including humans, livestock, and wild animals. M. bovis infection of wild boar has been reported in several European countries. We report here the first case of M. bovis infection in a domesticated wild sow in Korea. Granulomatous and necrotizing lesions with small numbers of acid-fast bacilli were observed in nodules of the lung of wild sow. Furthermore, the M. bovis isolate from the wild sow had spoligotype SB0140 and a novel MIRU-VNTR allelic profile, which is not found in cattle and deer in Korea.
Mycobacterium (M.) bovis is the main causative agent of tuberculosis, a chronic contagious disease in a broad range of mammals, including cattle, deer, llamas, pigs, cats, wild cats, foxes, possums, and badgers [28]. The possum and badger are considered maintenance hosts of M. bovis in New Zealand, as well as in the United Kingdom, Spain, Portugal, Ireland and France [7], whereas wild boar is considered a maintenance host of M. bovis in Spain, Portugal and the United Kingdom [169].
In Korea, M. bovis infection has been reported in cattle and deer, but not in wild boar. Here, we report the first M. bovis infection in a domesticated wild sow (Sus scrofa) in Korea.
In February 2012, an adult wild sow weighing approximately 200 kg was sent to the Veterinary Laboratory Agency, where a postmortem examination was performed by the authorized personnel according to the National Bovine Tuberculosis Eradication Programme in Korea. At necropsy, mandibular, retropharyngeal, bronchial, hilar and mediastinal lymph nodes and lungs were collected for histopathological examination and mycobacterial culture. Tissue samples for histopathology were fixed in 10% neutral buffered formalin, and sections were stained with hematoxylin and eosin (H&E) and Ziehl–Neelsen stain. Mycobacterial culture was performed using the BD BACTEC Mycobacteria Growth Indicator Tube (MGIT) 960 System, and culture isolates were identified using Ziehl-Neelsen stain and multiplex polymerase chain reaction (PCR) assay as described by Kim et al. [5]. The molecular type was determined using spoligotyping and the mycobacterial interspersed repetitive unit-variable number tandem repeat (MIRU-VNTR) method [34].
The nodules in the lung were very firm (panel A in Fig. 1), and their cut surface revealed thick yellow caseous material. The histopathology of lymph node sections revealed granulomatous and necrotizing lesions with small numbers of acid-fast bacilli (panel B in Fig. 1).
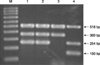
M. bovis was isolated and identified using multiplex PCR assay (Fig. 2). The spoligotype was determined as SB0140, and the MIRU-VNTR allelic profile was 253264103433101 based on the MIRU 2, MIRU 26, MIRU 27, MIRU 31, ETR-A, ETR-B, QUB 11a, QUB 18, QUB 26, VNTR 2401, VNTR 3171, VNTR 3232, and VNTR 3336 loci.
In Korea, wild swine are bred for meat. The M. bovis-infected wild sow was a brood sow, the offspring of a captured wild swine. Interestingly, the M. bovis isolate had a unique MIRU-VNTR allelic profile that has not been reported in other animals in Korea [3]. This implies that M. bovis strain in the sow might have originated from wild swine, but not from other livestock, such as cattle and deer. However, additional studies are needed to investigate whether M. bovis transmits between wild swine and domesticated pigs.
In conclusion, this report provides the first case of tuberculosis by M. bovis in a wild sow in Korea. The lymph node sections contained granulomatous and necrotizing lesions with acid-fast bacilli. M. bovis isolate from a wild sow had a novel MIRU-VNTR allelic profile.
Figures and Tables
Fig. 1
Visible lesions and Ziehl-Neelsen staining of the lungs of wild sow. (A) Visible lesions in the nodules of the lung from a wild sow showing well-demarcated yellow caseous nodules (arrowheads) at the cut surface. (B) Ziehl–Neelsen staining of a lung section nodule shows acid-fast bacilli (arrows) in a granuloma. Scale bar = 10 µm.
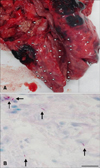
Fig. 2
Agarose gel electrophoresis of the polymerase chain reaction products using primers for the rpoB gene (518 bp), RD8 (Mycobacterium [M.] bovis and M. bovis BCG, 360 bp; M. tuberculosis, 150 bp), and RD1 (254 bp). Lane M, 100-bp DNA size marker; Lane 1, wild boar isolate; Lane 2, M. bovis AN5; Lane 3, M. bovis BCG Pasteur; Lane 4, M. tuberculosis H37Rv.
Acknowledgments
This work was supported by a grant from the Animal and Plant Quarantine Agency and in part by a grant from the Bio-industry Technology Development Program (grant No. 314025-03) of the Ministry of Agriculture, Food and Rural Affairs, Korea.
References
1. Barasona JA, Latham MC, Acevedo P, Armenteros JA, Latham ADM, Gortazar C, Carro F, Soriguer RC, Vicente J. Spatiotemporal interactions between wild boar and cattle: implications for cross-species disease transmission. Vet Res. 2014; 45:122.

2. Fitzgerald SD, Kaneene JB. Wildlife reservoirs of bovine tuberculosis worldwide: hosts, pathology, surveillance, and control. Vet Pathol. 2013; 50:488–499.

3. Je S, Ku BK, Jeon BY, Kim JM, Jung SC, Cho SN. Extent of Mycobacterium bovis transmission among animals of dairy and beef cattle and deer farms in South Korea determined by variable-number tandem repeats typing. Vet Microbiol. 2015; 176:274–281.

4. Jeon BY, Je S, Park J, Kim Y, Lee EG, Lee H, Seo S, Cho SN. Variable number tandem repeat analysis of Mycobacterium bovis isolates from Gyeonggi-do, Korea. J Vet Sci. 2008; 9:145–153.

5. Kim Y, Choi Y, Jeon BY, Jin H, Cho SN, Lee H. A simple and efficient multiplex PCR assay for the identification of Mycobacterium genus and Mycobacterium tuberculosis complex to the species level. Yonsei Med J. 2013; 54:1220–1226.

6. Naranjo V, Gortazar C, Vicente J, de la Fuente J. Evidence of the role of European wild boar as a reservoir of Mycobacterium tuberculosis complex. Vet Microbiol. 2008; 127:1–9.

7. Palmer MV. Mycobacterium bovis: characteristics of wildlife reservoir hosts. Transbound Emerg Dis. 2013; 60:Suppl 1. 1–13.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download