Abstract
Pelvic inflammatory disease (PID), which is one of the most problematic complications experienced by women with sexually transmitted diseases, frequently causes secondary infections after reproductive abnormalities in veterinary animals. Although the uterus is self-protective, it becomes fragile during periods or pregnancy. To investigate PID, bacteria or lipopolysaccharide (LPS) extracted from gram negative bacteria has been used to induce the disease in several animal models. However, when LPS is applied to the peritoneum, it often causes systemic sepsis leading to death and the PID was not consistently demonstrated. Hydrochloric acid (HCl) has been used to induce inflammation in the lungs and stomach but not tested for reproductive organs. In this study, we developed a PID model in mice by HCl and LPS sequential intracervical (i.c.) administration. The proinflammatory cytokines, interleukin (IL)-1β, IL-6 and tumor necrosis factor-α, were detected in the mouse uterus by western blot analysis and cytokine enzyme-linked immunosorbent assay after HCl (25 mg/kg) administration i.c. followed by four LPS (50 mg/kg) treatments. Moreover, mice exhibited increased infiltration of neutrophils in the endometrium and epithelial layer. These results suggest that ic co-administration of HCl and LPS induces PID in mice. This new model may provide a consistent and reproducible PID model for future research.
Pelvic inflammatory diseases (PID) are infections of the female reproductive organs that are one of the most serious complications experienced by women with sexually transmitted diseases. In veterinary animals, PID is the most frequent uterine disease following any abnormal parturition in cows and mares, such as an abortion, retained placenta, twin births, dystocia, and traumatic lacerations of the genital canal. Histologically, tissues afflicted by PID show severe infiltration of neutrophils accompanied by macrophages which proceeds accumulation of pus in the uterine lumen [715]. However, the normal nonpregnant uterus is endowed with a high degree of resistance to infection, which prevents bacterial growth and bacterial persistence for extended periods [16]. Uterine resistance varies during the estrous cycle and the susceptibility is greater during the luteal phase of the cycle. Some known factors make the uterus susceptible to infection but little is known about the mechanism of its resistance. Female genital canal infections are an important cause of infertility, pre-term labor and chronic pelvic pain in women [18] and a further cause of economic loss in the livestock industry, including reduced fertility, subsequent milk withdrawal and mortality [1117].
To find more efficient treatment options for PID, it is necessary to develop a further understanding of its pathogenesis and the self-limiting nature of the uterus toward ascending infection through experimental disease models. Disease models have been established in mice via direct live bacterial intrauterine (i.u.) infusion under laparotomy incision [134918] or in vitro using lipopolysaccharide (LPS), the most potent antigenic molecule of gram negative bacteria [12]. Disease models also reported that a single treatment of LPS induced inflammation of the genital tract of laboratory animals in several studies [57]. However, establishment of the PID model using live bacterial infusion such as Clostridium sordellii caused systemic sepsis in experimental animals, and the use of pathogenic live bacteria can potentially endanger researchers' health. LPS treatment also caused sepsis, leading to death, or could not induce the disease, depending on dosage or treatment route (unpublished preliminary data).
There have been efforts to develop effective animal models for studying inflammatory responses in a female reproductive organ. However, PID animal models that employ in situ pathogenic bacteria infusion via surgical intervention pose a risk to the animal and the researcher [18], or require powerful immunogenic bacterial components, such as LPS injection via intraperitoneal (i.p.) or intracervical (i.c.) routes. LPS i.p. administration into animals can cause systemic symptoms [19], dose dependent sepsis [613], and in some cases lead to death with inflammatory pain [2].
Thus, because hydrochloric acid (HCl) induced inflammation of macrophages and epithelial cells in vivo [10] and increased mucosal permeability in vitro [14], this study was conducted to develop the PID animal model in nonpregnant mice without i.u. infection by live pathogenic bacteria instead using HCl-LPS co-administration. The inflammatory reaction was assessed by determining proinflammatory cytokines in uterine tissue via western blot analysis and evaluating the histopathological findings.
LPS (Salmonella enterica serotype enteritidis), pregnant mare serum gonadotropin (PMSG), and a cocktail of proteinase inhibitors and antibody for β-actin were purchased from Sigma-Aldrich (USA). Hydrochloric acid was purchased from Yakuri Pure Chemicals (Japan). Dulbecco's Modified Eagle Medium (DMEM) and fetal bovine serum (FBS) were purchased from HyClone (USA) and penicillin-streptomycin was obtained from WELGENE (Korea). Antibodies for interleukin (IL)-1β and IL-6 were purchased from R&D Systems (USA) and tumor necrosis factor (TNF)-α was obtained from Cell Signaling Technology (USA).
Female C57BL/6J mice (8–10 weeks) weighing 20 to 22 g were obtained from Nara Biotech (Korea), maintained under a 12:12-h, light-dark cycle in a temperature and humidity controlled room (24 ± 5℃; 50 ± 10%) and fed on standard laboratory diet and water ad libitum.
To induce PID, groups were divided into five single treatments, two combined treatments, one negative control group and one positive control group. The single treatment groups were administered with 100 µL of LPS alone (25 mg/kg or 50 mg/kg), once intraperitoneally (LPS25ip, LPS50ip) or four times intracervically (LPS25ic, LPS50ic) or one time of HCl alone (25 mg/kg, 1 N) i.c. In the combined treatment groups, animals received one dose i.c. of HCl (25 mg/kg, 1 N), followed by four administrations of LPS (25 mg/kg or 50 mg/kg; HCl-LPS25, HCl-LPS50) every 2 h. Animals from groups treated via the i.c. route received 100 µL of PMSG (7.5 IU per mouse) two days before the experiment to induce proceptivity. Control mice received saline. For histopathological analysis, uterine tissue from the two uterine horns and the vagina were collected every 2 h until 10 h post initial inoculation. Mice were sacrificed using a CO2 gas chamber filled with 2 liters/min for 3 to 5 min and then submitted to cervical dislocation. All experimental procedures were performed in accordance with the guidelines established by the Kangwon National University Institutional Animal Care and Use Committee (KW-141231-2).
Mice were monitored every 2 h until 10 h post initial inoculation for clinical sign assessment according to the criteria shown in Table 1. Observers were blinded to treatment status.
RAW 264.7 cells were grown in DMEM supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin and 100 µg/mL streptomycin at 37℃ with 5% CO2. The cells (5 × 105 cells/mL) were cultured in the presence of LPS for 2 h in 12-well plates. Protein extracts were used as a positive control.
After mice were euthanized, the uterine tissues were frozen in liquid nitrogen immediately after removal and stored at –70℃ until use. A 5 mg piece of tissue was immediately added to a tube containing 300 µL of lysis buffer, homogenized with an electric homogenizer on ice and rinsed twice with another 2 × 300 µL lysis buffer. Sample was then maintained at constant agitation for 2 h at 4℃. After agitation, the sample tube was centrifuged for 20 min at 16,000 × g at 4℃ and kept on ice. The supernatant was transferred to a new tube that was also maintained on ice. Protein contents in the supernatant of the lysed tissue were determined using a Quick Start Bradford protein assay kit (Bio-Rad Laboratories, USA) according to the manufacturer's instructions. The pellet was discarded.
Samples were separated on 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes. After blocking with 5% skim milk in TBST (0.1%) for 3 h at room temperature, the membranes were incubated with a primary antibody (β-actin, 1:5,000; IL-1β, 1:500; IL-6, 1:500; TNF-α, 1:500), then incubated with the conjugated secondary antibodies. Protein bands were detected using an ECL detection kit (Lumigen ECL Plus Western Blotting HRP Substrate; Lumigen, USA). The protein signals were quantified by scanning densitometry using a C-DiGit Blot Scanner 3600 (ver. 3.1.4; LI-COR, USA).
For cytokine determination in uterine mucosa, protein extracts were isolated by homogenization of uterine tissue segments (50 mg tissue/mL) in 50 mmol/L Tris-HCl at pH 7.4 with 0.5 mmol/L dithiothreitol and 10 g/mL of a cocktail of proteinase inhibitors. Samples were centrifuged at 30,000 × g for 20 minutes, then stored at –70℃ until cytokine determination. Cytokine levels in the protein extracts from uterine tissues were determined by specific sandwich enzyme-linked immunosorbent assays (ELISA) using capture/biotinylated detection antibodies for IL-1β, IL-6 and TNF-α from R&D Systems according to the manufacturer's recommendations.
At the end of the experiment, all mice were euthanized and the uteruses were excised. A part of each uterus was fixed in 10% (w/v) neutral buffered formalin for 24 to 48 h, processed routinely and embedded in paraffin wax. Three sections (4 µm) were cut from each of the three blocks of tissue samples and stained with hematoxylin and eosin (H&E) for light microscopic examination. For morphometric analysis, six microscopic fields of each section were randomly selected. The histopathological score was determined "blindly" according to criteria shown in Table 2 and the number of neutrophils per unit area (0.25 mm2) was determined. To obtain quantitative data, the lesions were analyzed using Image J 1.43m Program (National Institute of Health, USA).
Summary statistics were calculated for all of the groups to assess the overall quality of the data including normality. Continuous data (cytokine levels) were analyzed by one-way analysis of variance (ANOVA). If the results of one-way ANOVA were significant, pairwise testing using Tukey's adjustment was performed. Discrete data (clinical evaluation and histopathological score) were analyzed by a Chi-squared and/or Fisher's exact test. Pearson's correlation test was used to analyze the relationship between the number of infiltrated neutrophils in the uterus and other test parameters, including the pro-inflammatory cytokine expression, clinical sign scores and histopathological scores. A value of p < 0.05 was considered significant.
The most commonly observed clinical signs were a hunched posture with ruffled hair and closed eyes. Clinical signs were observed in the LPS25ip and LPS50ip group as early as 2 hpi, peaked at 6 hpi and decreased between 8 and 10 hpi. HCl-LPS50 and HCl-LPS25 groups started to show clinical signs at 6 hpi HCl and LPS inoculation, and were quickly in remission between 8 and 10 hpi. LPS25ic, LPS50ic and HCl25 groups did not exhibit severe clinical signs. The mean scores of clinical signs were significantly higher in LPS50ip and LPS25ip groups (p < 0.001) than other groups. The mean scores of clinical signs in LPS50ic, LPS25ic and HCl25 groups were not significantly different between the groups (Fig. 1).
To investigate the inflammatory cytokine expression levels, IL-1β, IL-6, and TNF-α were evaluated in inflammation induced uterine tissues. Western blot analysis revealed that no cytokines were present in the uterine tissues from any of the single agent treated groups with various administration schemes, including LPS25ip, LPS50ip, LPS25ic, LPS50ic, and HCl25 and one combined treatment group, HCl-LPS25 (data not shown). Additionally, all tested cytokines were detected only in the HCl-LPS50 combined treatment group (Fig. 2).
HCl-LPS combined treatment induced significantly higher levels of IL-1β, IL-6 and TNF-α in uteruses (p < 0.001). In other groups, IL-1β and IL-6 levels were detected in trace amounts, but no significant differences were observed, rather, the TNF-α level was significantly upregulated in LPS25ip, LPS50ip, LPS25ic, and LPS50ic treated groups (p < 0.05; Fig. 3).
Uterine lesions in HCl-LPS50 (panel B in Fig. 4) and HCl-LPS25 (less intense; data not shown) were characterized by neutrophils infiltration, epithelial cell necrosis and degeneration, congestion and hemorrhage. In the other groups (LPS25ip, LPS50ip and HCl25), slight histopathological findings including congestion related edema and negligible infiltration of neutrophils were observed.
The scores of uterine lesions and numbers of infiltrated neutrophils were significantly higher in the combined treated HCl-LPS50 and HCl-LPS25 groups (p < 0.001) than other groups, followed by single treated LPS50ic and LPS25ic groups (p < 0.05) and then the remaining groups (Figs. 5 and 6). The number of infiltrated neutrophils was correlated with pro-inflammatory cytokine expression in uterine tissue (TNF-α, r = 0.853, p < 0.001; IL-1β, r = 0.798, p < 0.001; IL-6, r = 0.785, p < 0.001) and histopathological scores (r = 0.669, p < 0.001), but not correlated with clinical sign scores (r = 0.293, p = 0.069).
We conducted this study to establish a PID animal model for pelvic inflammatory diseases (PIDs) to further contribute to PID treatment options. PIDs have serious complications for genital health in both humans and animals, especially in domestic animals. Indeed, these diseases are directly connected to economic losses because of resultant infertility, increased culling for failure to conceive, reduced production, and increased costs for drug treatments [51118].
However, LPS is widely used to stimulate inflammation in vivo and in vitro in various tissues and organs, and because it can activate monocytes in blood and histiocytes in tissue to secrete the pro-inflammatory cytokines, IL-1β, IL-6, IL-8, and TNF-α [20], which are involved in the inflammatory cascade and increase body temperature, pain, and the production of other inflammatory proteins [13].
In a preliminary study to determine whether PID was installed via i.p. or i.c. LPS alone, we investigated the cytokine expression levels in uterine tissue through western blot from 12 to 48 hpi; however, we could not find any trace of cytokines from those groups (data not shown). Rather, we observed acute inflammatory responses such as fever, shivering, and lethargy accompanying general vital impairments as previously described. The dose of LPS 50 mg/kg (LPS50ip) administered i.p. was an even higher dose than that used to cause septic death in Balb/c mice at 24 hpi [2]. Deb et al. [7] observed increased IL-1β causing disturbance of the intrauterine implantation and macrophage infiltration into the uterus with a single dose of 5 µg per animal on day 0.5 of pregnancy. However, the study was performed in a pregnant uterus, which are more susceptible to infection than nonpregnant uteruses. These findings indicate that single treatment with LPS causes uterus inflammation, although only when administered in high dose.
To avoid LPS related systemic effects and only induce pelvic inflammation, intra-uterine infusions of Clostridium sordellii [3], Escherichia coli [18], and LPS [5] were attempted by either laparotomy incision [318] or i.c. injection [5]. Pathogenic bacterial infection caused mortalities if the dose was not cautiously adjusted; however, single administration of LPS i.c. successfully presented leukocyte infiltration into the uterus without any systemic reaction.
As uterine epithelial cells provide a physical barrier and defend underlying tissues from invading pathogens [8], we first used HCl to induce injury [1420], after which LPS was administered i.c. The animals modeled by this method presented strongly pro-inflammatory cytokine expressions, such as IL-1β, IL-6, and TNF-α, via either western blot analysis or ELISA in their uterine tissue with reduced systemic inflammatory symptoms compared with animals treated with LPS administered alone i.p. or i.c. Interestingly, neutrophil infiltration into the uterus had a high correlation with pro-inflammatory cytokine expressions and histopathological scores, but not with clinical signs.
It has been suggested that LPS could act more directly on the endometrium, including epithelial and stromal cells of the uterus and resident macrophages, to produce high levels of cell-alarming cytokines as the physical defense mechanism was disrupted by HCl. Moreover, histopathological evaluation revealed severe immune cell infiltration, degeneration of uterine glands, and epithelial hyperplasia, indicating that the PID model developed in this study was consistent with that of other PID models [1518].
In conclusion, we demonstrate here for the first time that initial i.c. treatment of HCl followed by four LPS applications could establish a PID animal model without surgical intervention, acute systemic reaction, or posing safety risks to the researcher. Furthermore, the combined use of HCl and LPS could be a useful model for understanding the mechanisms by which innate physical barriers should be impaired before LPS binds to TLR4 receptor in resident immune and endometrial, epithelial and stromal cells to stimulate the synthesis and production of proinflammatory cytokines.
Figures and Tables
Fig. 1
Mean value of clinical sign scoring. ***Significantly more serious clinical signs in lipopolysaccharide (LPS) intraperitoneal administered animals compared with LPS or hydrochloric acid (HCl)-LPS intracervical (i.c.) administered animals (p < 0.001).

Fig. 2
Changes in cytokine expression in uterine tissue after co-administration of HCl and LPS. (A) Mice were administered i.c. with HCl (25 mg/kg) followed by four applications of LPS (25 mg/kg or 50 mg/kg) every 2 h. Two hours after the final injection, the uterus was removed and protein expression was assessed by Western blot. (B) Mice were administered i.c. with HCl (25 mg/kg, 1 N) followed by four applications of LPS (50 mg/kg) every 2 h.

Fig. 3
Bar graphs for inflammatory cytokines measured by enzyme-linked immunosorbent assay in the uterine tissues of different experimental groups and a normal control. *Significantly higher inflammatory cytokine levels than the HCl25 and Neg CTL groups (p < 0.05). ***Significantly higher inflammatory cytokine levels in the uteruses of HCl-LPS combined with i.c. administered animals (p < 0.001).

Fig. 4
The representative histopathological findings of the uterus with saline only (A) and co-administration of HCl and LPS (B). (B) Mice were administered i.c. with HCl (25 mg/kg. 1 N) followed by four applications of LPS (50 mg/kg) every 2 h. Note the neutrophils in the lumen, in the endometrium and in the surface epithelial layer (arrows). No inflammation was observed in (A). L, lumen. H&E stain. 200×.
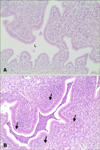
Fig. 5
Bar graphs for histopathological scoring in different experimental groups. *Significantly higher histopathological scores in LPS25ic and LPS50ic groups than in LPS25ip, LPS50ip, HCl25, and Neg CTL groups (p < 0.05). ***Significantly higher histopathological scores in HCl-LPS25 and HCl-LPS 50 groups than with other groups (p < 0.001).
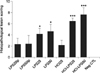
Fig. 6
Bar graphs for the number of infiltrated neutrophils per unit area (0.25 mm2) in uterine tissue of different experimental groups. *Significantly increased number of neutrophils in LPS25 and LPS50 groups than in LPS25ip, LPS50ip, and Negative control groups (p < 0.05). ***Significantly increased number of neutrophils in HCl-LPS25 and HCl-LPS 50 groups compared with other groups (p < 0.001).

Acknowledgments
This research was supported by the Bio-industry Technology Development Program (grant No. 112130-3) funded by the Ministry for Food, Agriculture, Forestry and Fisheries of the Republic of Korea, and by a 2014 Research Grant from Kangwon National University and technical support from the Institute of Veterinary Medicine in Kangwon National University, Korea.
References
1. Abdullah FFJ, Adamu L, Ismael NA, Osman AY, Haron AW, Saad MZ, Saharee AA. Clinical responses and reproductive pathological changes associated with Brucella melitensis and it's lipopolysaccharides in female mice. Int J Anim Vet Adv. 2014; 6:15–22.

2. Ando H, Takamura T, Ota T, Nagai Y, Kobayashi K. Cerivastatin improves survival of mice with lipopolysaccharide-induced sepsis. J Pharmacol Exp Ther. 2000; 294:1043–1046.
3. Aronoff DM, Hao Y, Chung J, Coleman N, Lewis C, Peres CM, Serezani CH, Chen GH, Flamand N, Brock TG, Peters-Golden M. Misoprostol impairs female reproductive tract innate immunity against Clostridium sordellii. J Immunol. 2008; 180:8222–8230.

4. Bernstein-Hanley I, Coers J, Balsara ZR, Taylor GA, Starnbach MN, Dietrich WF. The p47 GTPases Igtp and Irgb10 map to the Chlamydia trachomatis susceptibility locus Ctrq-3 and mediate cellular resistance in mice. Proc Natl Acad Sci U S A. 2006; 103:14092–14097.

5. Brecchia G, Menchetti L, Cardinali R, Castellini C, Polisca A, Zerani M, Maranesi M, Boiti C. Effects of a bacterial lipopolysaccharide on the reproductive functions of rabbit does. Anim Reprod Sci. 2014; 147:128–134.

6. Chen G, Li J, Qing X, Czura CJ, Ochani M, Ochani K, Ulloa L, Yang H, Tracey KJ, Wang P, Sama AE, Wang H. Suppression of HMGB1 release by stearoyl lysophosphatidylcholine: an additional mechanism for its therapeutic effects in experimental sepsis. J Lipid Res. 2005; 46:623–627.

7. Deb K, Chaturvedi MM, Jaiswal YK. Gram-negative bacterial LPS induced poor uterine receptivity and implantation failure in mouse: alterations in IL-1β expression in the preimplantation embryo and uterine horns. Infect Dis Obstet Gynecol. 2005; 13:125–133.

8. Fahey JV, Wright JA, Shen L, Smith JM, Ghosh M, Rossoll RM, Wira CR. Estradiol selectively regulates innate immune function by polarized human uterine epithelial cells in culture. Nucosal Immunol. 2008; 1:317–325.

9. Fang L, Nowicki BJ, Dong YL, Yallampalli C. Localized increase in nitric oxide production and the expression of nitric oxide synthase isoforms in rat uterus with experimental intrauterine infection. Am J Obstet Gynecol. 1999; 181:601–609.

10. Jiang Y, Wang MH. Ethanol extract of Synurus deltoids (Aiton) Nakai suppresses in vitro LPS-induced cytokine production in RAW 264.7 macrophages and in vivo acute inflammatory symptoms. Nutr Res Pract. 2014; 8:11–19.

11. Laven RA, Peters AR. Bovine retained placenta: aetiology, pathogenesis and economic loss. Vet Rec. 1996; 139:465–471.

12. Luo J, Xu Y, Zhang M, Gao L, Fang C, Zhou C. Magnolol inhibits LPS-induced inflammatory response in uterine epithelial cells. Inflammation. 2013; 36:997–1003.

13. Moore CC, Martin EN, Lee G, Taylor C, Dondero R, Reznikov LL, Dinarello C, Thompson J, Scheld WM. Eukaryotic translation initiation factor 5A small interference RNA-liposome complexes reduce inflammation and increase survival in murine models of severe sepsis and acute lung injury. J Infect Dis. 2008; 198:1407–1414.

14. Nylander O, Holm L, Wilander E, Hällgren A. Exposure of the duodenum to high concentrations of hydrochloric acid: effects on mucosal permeability, alkaline secretion, and blood flow. Scand J Gastroenterol. 1994; 29:437–444.

16. Schlafer DH, Miller RB. Female genital system. In : Maxie MG, editor. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 5th ed. London: Elsevier;2007. p. 466–474.
17. Sheldon IM, Lewis GS, LeBlanc S, Gilbert RO. Defining postpartum uterine disease in cattle. Theriogenology. 2006; 65:1516–1530.

18. Sheldon IM, Rycroft AN, Dogan B, Craven M, Bromfield JJ, Chandler A, Roberts MH, Price SB, Gilbert RO, Simpson KW. Specific strains of Escherichia coli are pathogenic for the endometrium of cattle and cause pelvic inflammatory disease in cattle and mice. PLoS One. 2010; 5:e9192.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download