Abstract
This study was conducted to establish accurate baseline values of clinical laboratory data with regard to age-related changes in the Oriental white stork (Ciconia boyciana). In addition, the availability of an automated hematological cell counter was evaluated. A total of 94 clinically normal storks, including 64 young storks (<1 year old; 30 male and 34 female) and 30 adults (> 1 year old; 17 male and 13 female) were included. Hematological assays were performed using manual and automated cell counters and serum biochemistry profiles were examined using an automated analyzer. There were no significant differences in any parameters between male and female storks, while 16 parameters were significantly different between young and adult storks. Of these 16 parameters, total protein, albumin, aspartate aminotransferase, alanine aminotransferase, creatinine, triglyceride, total bilirubin, potassium, white blood cell count, packed cell volume, mean cell volume and hemoglobin levels were higher in adult storks than in young storks, while the latter showed higher glucose, uric acid and alkaline phosphatase levels, as well as a higher sodium/potassium ratio. The results presented herein will aid researchers who work for the conservation and rehabilitation of this endangered species.
The Oriental white stork (Ciconia [C.] boyciana) belongs to the family Ciconiidea and the order Ciconiformes. This bird can be found in Japan, China, Korea, and Russia, although it is no longer a permanent resident in South Korea. Currently, this stork mainly inhabits the Heilong River and Wusuli River basins along the border between Russia and China [27]. The International Union for Conservation of Nature considers C. boyciana an endangered species. The Korea Institute of Oriental white stork Rehabilitation Research at Korea National University of Education is attempting to reintroduce this species as a breeding bird in Korea.
Blood analyses are widely used to diagnose and monitor general health and disease along with physiological processes in wild and captive birds as part of efforts to increase captive populations [6112331]. In addition, wild birds tends to hide clinical signs of disease; therefore, observations by the keeper should be complemented by periodic blood analyses if sick or injured birds are to be diagnosed and treated in the early stages of disease [1417]. While interpreting data pertaining to blood analyses for animals, age must be considered because of significant differences between young and adult individuals [2918]. Previous studies have reported hematological and biochemical parameters for some species of storks [192226]; however, to the best of our knowledge, baseline and age-related changes in these parameters for young and adult C. boyciana have not been published to date.
Therefore, this study was conducted to establish accurate baseline values of clinical laboratory data for C. boyciana with regard to age- and sex-related changes. In addition, the availability of an automated hematological cell counter was evaluated for rapid and accurate examination of the C. boyciana population [3724]. These data will help researchers who work for the conservation and rehabilitation of this endangered species.
A total of 94 clinically normal storks, including 64 young (< 1 year old; 30 male and 34 female) and 30 adult (> 1-year old; 17 male and 13 female) birds, were evaluated in this study. All storks showed visually normal behavior and appetite, as determined by experienced keepers and veterinarians, and were presexed through phenotyping and DNA tests as described by Han et al. [12]. All young storks evaluated in this study were born and raised in the Korea Institute of Oriental white stork Rehabilitation Research. During the experiment, the storks were housed individually in a 7 × 7 m outdoor cage with chain-link fencing. Each cage was covered with netting, and a pond and roost were established on the ground of the cage.
Peripheral blood was collected from the caudal tibial vein of the storks using a 24-gauge needle. The collected blood was placed into heparin-treated tubes and serum separator tubes, and serum was obtained from the clotted blood in the serum separator tube after centrifugation at 1,500 × g for 5 min.
A manual hematological examination was performed to determine the packed cell volume (PCV), hemoglobin (Hb) level, red blood cell (RBC) and white blood cell (WBC) counts, and the WBC differential count [29]. The WBC and RBC counts were determined using a hemocytometer and Natt–Herick's solution. Microhematocrit tubes coated with heparin were filled with blood and centrifuged at 1,500 × g for 5 min to determine the PCV. Hb was assayed by the cyanmethemoglobin method. The mean cell volume (MCV), mean cell Hb (MCH), and mean cell Hb concentration (MCHC) were calculated using the following formulae: MCV = (PCV/RBC) × 10; MCH = (Hb/RBC) × 10; and MCHC = (Hb/PCV%) × 100. An air-dried blood smear was stained with Diff-Quik stain (Sysmex, Kobe, Japan), and a manual 100 cell differential count was obtained.
Serum biochemistry profiles were obtained using a 7020 Automatic analyzer (Hitachi High-Technologies, Japan). The assay included total protein, albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase, blood urea nitrogen (BUN), creatinine, total cholesterol, triglyceride, glucose, total bilirubin, creatine phosphokinase (CPK), amylase, lactate dehydrogenase (LDH), uric acid, total calcium, phosphorus, magnesium, sodium, potassium, and chloride levels. The albumin/globulin (A/G) ratio and sodium/potassium (Na/K) ratio were calculated based on the measured values of each parameter.
Samples from 16 randomly selected adult storks were analyzed using the Cell-Dyn 3700 (Abbott Laboratories, USA) to evaluate the potential for use of the machine as an automatic hematological analyzer. For this evaluation, the analyzer was adjusted with the veterinary package software. Because of the presence of nucleated RBCs in birds, the time for hemolysis in the analyzer was adjusted to a longer incubation time (34 sec). The WBC count was measured using both the white cell impedence count (WIC) and white cell optical count (WOC) methods, while the RBC count and RBC-related parameters were measured using the WIC method.
Differences in variables between the young and adult groups or the manual and automated methods were analyzed using an independent t-test. Differences in variables between the male and female storks in the young and adult groups were analyzed using one-way ANOVA. The Kolmogorov–Smirnov test (p < 0.05) was used to determine if the data had a Gaussian distribution [20]. When data were from a Gaussian distribution, reference intervals were defined by minimum and maximum values for groups of fewer than 40 samples and by central 95% percentiles (mean ± 2SD) for groups of more than 40 samples [28]. For results that did not significantly differ between groups, reference intervals were determined from values pooled from two groups of storks [52028]. All statistical analyses were conducted using SPSS statistical software for Windows (ver. 18.0.0.0; SPSS, USA). A p value of < 0.05 was considered statistically significant.
The agreement between assessment methods for hematological parameters was evaluated using the Passing-Bablok regression analysis and Bland-Altman difference plots. The agreement between two methods was considered good when the 95% confidence interval included the value 0 for the intercept and the value 1 for the slope. If one or both of the values did not satisfy these criteria, the agreement was considered poor [24].
No significant differences were observed in hematological parameters between male and female storks in both the young and adult groups (i.e., young male versus adult male and young female versus adult female, respectively). Upon evaluation of age-related differences, the WBC count, PCV, Hb level, and MCV were significantly higher in adult storks than in young storks (p < 0.001 for both parameters), while none of the hematological parameters were higher in young storks (Table 1). Hematological reference intervals for storks are given in Table 1.
Serum biochemistry profiles did not differ significantly between male and female storks in the young or adult groups (i.e., young male versus adult male and young female versus adult female, respectively). Evaluation of age-related differences revealed that total protein, albumin, AST, ALT, creatinine, triglyceride, total bilirubin, and potassium levels were significantly higher in adult storks, while young storks showed significantly higher glucose, uric acid, and ALP levels, and a higher Na/K ratio (Table 2). Reference intervals for the serum biochemistry profiles of this stork are given in Table 2.
There was good agreement between the results obtained using the manual method and those obtained using the analyzer for all hematological parameters (Table 3; Fig. 1). These findings were consistent with those of Bland–Altman plots, although the biases were small and the 95% confidence intervals were wide (Fig. 2).
Knowledge of the physiological state and causes of illness and death can improve and facilitate the selection and application of proper management strategies for the conservation of endangered species [101621]. Clinical hematology and serum biochemistry profiles are useful diagnostic tools in clinical practice that are particularly important for birds because they generally hide the clinical signs of disease [13]. The primary purpose of this study was to establish reference intervals for hematological and serum biochemical parameters in healthy young and adult captive storks. The results presented herein will be valuable to conservation and rehabilitation projects for the stork.
In the field of veterinary medicine, automatic hematological instruments are widely used in most specialized laboratories and hospitals to simplify hematological analysis [242532]. Unlike instruments used for serum biochemical parameters [1], the application of hematological instruments to bird species is somewhat complicated because of the presence of nucleated RBCs and thrombocytes [4]. Naked nuclei from lysed RBCs and nucleated thrombocytes are similar in size to lymphocytes; therefore, there is a false-positive increase in the leukocyte number in automatic cell counters when the WIC method is used alone. In contrast, the Cell-Dyn 3700 we used in this study uses both the WIC and WOC methods, providing two sets of results with an error message if both methods represent significantly different results [30]. Therefore, we evaluated this machine for hematological analysis of the Oriental white stork. Overall, the results demonstrated acceptable precision for every evaluated hematological parameter.
Among hematological parameters, WBC, PCV, and Hb levels, as well as MCV were higher in adults storks than in young storks. The results of RBC-related parameters may be due to the immature and developmental physiological states of young storks, which are similar to other bird species [81517]. Additionally, the results of the present study were similar to those of studies of other species of stork chicks (Ciconia ciconia) [2226]. However, the higher WBC count of the adult storks in this study was different from the results of other species of stork chicks. Rather, the age-related change in WBC count was similar to that of a study of the bearded vulture (Gypaetus barbatus), which showed higher WBC counts in adult groups [15].
Among serum biochemical parameters, eight (total protein, albumin, AST, ALT, creatinine, triglyceride, total bilirubin, and potassium) were higher in the adult storks than in young ones, which showed higher glucose, uric acid and ALP levels, as well as higher Na/K ratios. The difference in total protein and albumin levels may have been due to the immature and developmental physiologic states of young storks, which are similar to other bird species [81517]. The higher uric acid, ALP, and glucose levels found in young storks may have been caused by increased protein synthesis, increased bone metabolism, or higher energy requirements [17], whereas increased activities of other parameters (AST, ALT, creatinine, triglyceride, total bilirubin, and potassium) may have resulted from increased basal metabolism and tissue turnover [17]. Notably, none of these parameters showed sex-related differences in either the young or adult groups. These findings are inconsistent with those for the black stork C. nigra, which showed greater levels of Hb, total protein, ALP, and triglyceride in females, while males showed higher levels of albumin [19], probably reflecting interspecies variability.
In conclusion, this study determined hematological and serum biochemistry profiles for evaluating the health status of the Oriental white stork C. boyciana. We also demonstrated that the Cell-Dyn 3700, an automated cell counter, can provide accurate hematological data in a short period of time.
Figures and Tables
Fig. 1
Passing-Bablok scattergrams showing the agreement between hematological data obtained on manual count and using the Cell-Dyn 3700 for hematological parameters in the Oriental white stork (Ciconia boyciana). The solid red line represents the regression line, the solid gray lines represent the 95% confidence intervals, and the dotted line represents the identity line.

Fig. 2
Bland-Altman plots showing the agreement between the hematological data obtained on manual count and Cell-Dyn 3700 for hematological parameters in the Oriental white stork (Ciconia boyciana). X-axes represent the average for both methods, and Y-axes represent the difference between assessments.

Table 1
Difference in hematological parameters between young (n = 64) and adult (n = 30) Oriental white storks (Ciconia boyciana)
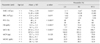
Table 2
Difference in parameters of serum biochemistry profiles between young (n = 64) and adult (n = 30) Oriental white storks (Ciconia boyciana)
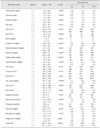
*Significantly different between young (< 1-year old) and adult (> 1-year old) groups. A/G, albumin/globulin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; CPK, creatine phosphokinase; LDH, lactate dehydrogenase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transpeptidase.
Acknowledgments
This study was supported by research funds for newly appointed professors of Chonbuk National University in 2015.
References
1. Ammersbach M, Beaufrère H, Gionet Rollick A, Tully T. Laboratory blood analysis in Strigiformes—Part II: plasma biochemistry reference intervals and agreement between the Abaxis Vetscan V2 and the Roche Cobas c501. Vet Clin Pathol. 2015; 44:128–140.

2. Bailey TA, Wernery U, Howlett J, Naldo J, Samour JH. Age-related plasma chemistry changes in houbara and kori bustards in the United Arab Emirates. J Wildl Dis. 1999; 35:31–37.

3. Bauer NB, Nakagawa J, Dunker C, Failing K, Moritz A. Evaluation of the impedence analyzer PocH-100iV Diff for analysis of canine and feline blood. Vet Clin Pathol. 2012; 41:194–206.

4. Beaufrère H, Ammersbach M, Tully TN Jr. Complete blood cell count in psittaciformes by using high-throughput image cytometry: a pilot study. J Avian Med Surg. 2013; 27:211–217.

5. Chung CS, Cheng CH, Chin SC, Lee AH, Chi CH. Morphologic and cytochemical characteristics of Asian yellow pond turtle (Ocadia sinensis) blood cells and their hematologic and plasma biochemical reference values. J Zool Wildl Med. 2009; 40:76–85.

6. Cooper JE. Minimally invasive health monitoring of wildlife. Anim Welf. 1998; 7:35–44.
7. Criswell KA, Bock JH, Wildeboer SE, Johnson K, Giovanelli RP. Validation of Sysmex XT-2000iV generated quantitative bone marrow differential counts in untreated Wistar rats. Vet Clin Pathol. 2014; 43:125–136.

8. Dujowich M, Mazet JK, Zuba JR. Hematologic and biochemical reference ranges for captive California condors (Gymnogyps californianus). J Zoo Wildl Med. 2005; 36:590–597.

9. Fudge AM. Clinical haematology and chemistry of ratites. In : Tully TN, Shane SM, editors. Ratite Management, Medicine and Surgery. 1st ed. Malabar: Krieger Publishing;1996. p. 214–245.
10. González LM, Margalida A, Mañosa S, Sánchez R, Oria J, Molina JI, Caldera J, Aranda A, Prada L. Causes and spatio-temporal variations of non-natural mortality in the vulnerable Spanish imperial eagle Aquila adalberti during a recovery period. Oryx. 2007; 41:495–502.

11. Han JI, Jang HJ, Lee SJ, Kang HM, Kim S, Park SR, Na KJ. Bacterial flora of the intestine in normal captive Oriental white storks. J Vet Clin. 2011; 28:516–518.
12. Han JI, Kim JH, Kim S, Park SR, Na KJ. A simple and improved DNA test for avian sex determination. Auk. 2009; 126:779–783.

13. Harr KE. Clinical chemistry of companion avian species: a review. Vet Clin Pathol. 2002; 31:140–151.

14. Hawkey CM, Dennet TB, Peirce MA. Color Atlas of Comparative Veterinary Hematology. London: Wolfe Medical;1989. p. 192.
15. Hernández M, Margalida A. Hematology and blood chemistry reference values and age-related changes in wild bearded vultures (Gypaetus barbatus). J Wildl Dis. 2010; 46:390–400.

16. Hernández M, Margalida A. Pesticide abuse in Europe: effects on the cinereous vulture (Aegypius monachus) population in Spain. Ecotoxicology. 2008; 17:264–272.

17. Hochleithner M. Biochemistries. In : Ritchie BW, editor. Avian Medicine: Principles, and Application. Lake Worth: Wingers;1994. p. 223–245.
18. Howlet JC, Bailey TA, Naldo JL. Age-related hematology changes in captive reared kori bustards (Ardeotis kori). Comp Haematol Int. 1998; 8:26–30.
19. Lanzarot MP, Barahona MV, San Andrés MI, Fernández-García M, Rodríguez C. Hematologic, protein electrophoresis, biochemistry, and cholinesterase values of free-living black stork nestlings (Ciconia nigra). J Wildl Dis. 2005; 41:379–386.

20. Lumsden JH, Mullen K. On establishing reference values. Can J Comp Med. 1978; 42:293–301.
21. Margalida A. Bearded vultures (Gypaetus barbatus) prefer fatty bones. Behav Ecol Sociobiol. 2008; 63:187–193.

22. Montesinos A, Sainz A, Pablos MV, Mazzucchelli F, Tesouro MA. Hematological and plasma biochemical reference intervals in young white storks. J Wildl Dis. 1997; 33:405–412.

23. Naidoo V, Diekmann M, Wolters K, Swan GE. Establishment of selected baseline blood chemistry and hematologic parameters in captive and wild-caught African white-backed vultures (Gyps africanus). J Wildl Dis. 2008; 44:649–654.

24. Perez-Ecija A, Gonzalez-De Cara CA, Aguilera-Aguilera R, Estepa JC, Rubio MD, Mendoza FJ. Comparison of donkey hemogram using the LaserCyte hematology analyzer, an impedence system, and a manual method. Vet Clin Pathol. 2014; 43:525–537.

25. Piviani M, Segura D, Monreal L, Bach-Raich E, Mesalles M, Pastor J. Neutrophilic myeloperoxidase index and mean light absorbance in neonatal septic and nonseptic foals. Vet Clin Pathol. 2011; 40:340–344.

26. Puerta ML, Munõz Pulido R, Huecas V, Abelenda M. Hematology and blood chemistry of chicks of white and black storks (Ciconia ciconia and Ciconia nigra). Comp Biochem Physiol A Comp Physiol. 1989; 94:201–204.

27. Smirenski SM. Oriental white stork action plan in the USSR. In : Coulter MC, Wang Q, Luthin CS, editors. Biology and Conservation of the Oriental White Stork (Ciconia boyciana). Aiken: Savannah River Ecology Laboratory;1991. p. 165–177.
28. Solberg HE. Establishment and use of reference values. In : Burtis CA, Ashwood ER, Tietz NW, editors. Tietz Textbook of Clinical Chemistry. 3rd ed. Philadelphia: WB Saunders;1999. p. 336–356.
29. Stockham SL, Scott MA. Fundamentals of Veterinary Clinical Pathology. 2nd ed. Ames: Wiley-Blackwell;2008. p. 62–124.
30. Suljević E, Fazlić M, Corić J, Kiseljaković JC. Evaluation of haematology analyzer Cell-Dyn 3700 SL. Bosn J Basic Med Sci. 2003; 3:35–41.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download