Abstract
A cross-sectional serological study was conducted in Shandong province of China to determine the seroprevalence and risk factors associated with seropositivity due to pseudorabies virus (PRV) infection in small- and medium-sized farrow-to-finish herds following outbreaks of variant PRV strains. A total of 6,035 blood samples from 224 randomly selected herds were screened. The results showed that 25.0% of the herds and 56.7% of the serum samples were seropositive for field strains of PRV. Herds consisting of 50–100 breeding sows had higher herd seroprevalence and serum sample seroprevalence than larger herds. Both the highest herd seroprevalence and highest serum sample seroprevalence were observed in western Shandong, followed northern Shandong. Based on univariate analysis, the following risk factors were utilized in subsequent multivariable logistic regression analysis: region, herd size, weight of purchased gilts, and all-in/all-out practice. Upon multivariate analysis, region, herd size, weight of purchased gilts and all-in/all-out practice were significantly associated with PRV herd seropositivity. These findings indicate that we are facing a serious situation in the prevention and control of pseudorabies. The results could help predict the next outbreak and set out control measures.
Pseudorabies (PR), also known as Aujeszky's disease, is an economically important viral disease worldwide, especially in developing countries [14]. PR is caused by pseudorabies virus (PRV; Suid herpesvirus 1), which can infect both livestock and wild animals, resulting in increased morbidity and mortality [16]. PRV can infect pigs at various production phases, and causes high mortality rates and nervous system disorders in newborn piglets, respiratory disorders in older pigs, and reproductive failure in pregnant sows, resulting in significant economic losses to the swine industry [2429].
For approximately 30 years, inactivated vaccines and, especially, live attenuated vaccines have been used to control outbreaks of PR, which has reduced the large economic losses associated with PRV [713]. By using glycoprotein E (gE)-deleted PRV vaccines, the United States and some European countries have eradicated PR from their domestic pig populations [310].
In China, an outbreak of PRV was first reported in the 1950s, and the Bartha-K61 vaccine was imported from Hungary to China in the 1970s. From the 1990s until late 2011, > 80% of pigs in China were vaccinated with the Bartha-K61 vaccine, and PR was well controlled [19]. However, since late 2011, a severe PR epidemic characterized by abortions and stillbirths of sows, as well as neurological signs and high-mortality among newborn piglets, has occurred in many pig herds immunized with live PRV vaccines in many regions of China [1142930]. It has been reported that the isolated PRV strains were highly pathogenic PRV antigenic variants with unique molecular signatures compared with those of classical PRV strains in China, the United States, and Europe [1714]. The epidemic quickly spread throughout northern China, resulting in a rapid increase in seropositivity for field strains of PRV. This primarily affected piglets and growing pigs and had a reported mortality rate of 10%–50%, while a later epidemic in southern China resulted in limited losses and seroconversion among swine herds in which PRV had been previously eradicated [11429].
According to the official statistics of the Shandong Statistical Bureau, there were about 36,585,900 pigs at the end of 2012 in Shandong, and the number of finishing pigs for slaughter per year was about 57,942,000. Densities exceeded 200 pigs/km2 in 11 of 17 cities in Shandong, and the average density was about 233 pigs/km2. Because of the high proportion of small- and medium-sized herds and the high regional breeding density in Shandong province, the prevalence of epidemic diseases is severe. This is a result of there being a background of ubiquitous immunosuppressive factors, such as mycotoxin and porcine circovirus type 2 [6]. Swine diseases, especially the variant PRV epidemic among Bartha-K61-vaccinated piglets since late 2011, have restricted the development of the swine industry in Shandong province, which has created great challenges for the prevention and control of swine diseases.
In Shandong, a mass-vaccination program has been implemented, and the marker vaccine has played a critical role in the control and eradication of PRV since the 1990s [1]. Marker vaccines that lack the gE gene do not induce anti-gE antibodies, as detected by a gE-specific enzyme-linked immunosorbent assay (ELISA); thus, it is possible to differentiate the vaccinated pigs from the infected ones, which can lead to eradication of field PRV strains [811]. Most pigs in Shandong are vaccinated according to the following schemes: sows and boars are vaccinated simultaneously three times per year with the gE-deleted PRV live vaccine. Piglets are vaccinated first through the intranasal route at 1‒3 days of age, then receive a second vaccination via an intramuscular injection at 8‒10 weeks of age. Replacement gilts and boars are subsequently vaccinated at 12‒16 weeks, which is followed by a supplementary immunization before estrus (unpublished data).
In this study, we determined the seroprevalence and risk factors associated with seropositivity due to PRV infections from January 2012 to August 2014 in Shandong province in four testing laboratories.
A cross-sectional study was conducted in 269 non-repetitively selected small- and medium-sized farrow-to-finish herds in Shandong. A total of 6,035 blood samples from 224 herds were screened to determine the seroprevalence and risk factors associated with seropositivity due to PRV infection. Blood samples for this study were randomly collected from the precaval vein according to a systematic sampling scheme (Table 1) from January 2012 to August 2014. The scheme can detect herds in which the PRV animal-level seroprevalence is 20% or higher with a 95% probability [25]. For each sample, the herd type, sample date, herd location, animal identification, gilts replacement policy, breeding methods, and production management were recorded. Serum was obtained after centrifugation of the coagulated blood samples and stored at -20℃ prior to testing.
Anti-gE antibodies in the serum samples were detected using the anti-gE antibody ELISA kit (IDEXX Laboratories, USA) according to the manufacturer's instructions. Serum samples with an S/N ratio ≤ 0.60 were considered to be positive for antibodies to the PRV gE antigen, and the presence of anti-gE antibodies indicates previous exposure to a field strain of PRV. A herd was considered to be infected when one or more samples were positive.
The following items obtained from previous reports and record charts were assumed to be risk factors: sampling year, region, herd size, gilts replacement policy, weight of purchased gilts, quarantine of purchased gilts, and all-in/all-out practice [25]. For each factor, between two and five categories were established.
The sero-status of a herd was coded as a dichotomous variable (positive/negative), and all variables from the record chart were defined as dichotomous variables or polytomous variables. We used an unconditional Pearson's chi-square test for dichotomous variables, and the chi-square test for both ordinal and continuous variables categorized by quartiles [2526]. If the expected number in one of the cells of the 2 × 2 tables was < 5, Fisher's exact probability test was used. Variables with a p < 0.1 based on the chi-square test were used in the subsequent multivariable logistic regression analysis.
The involvement of various factors in the seroconversion of PRV field strains was analyzed using multiple logistic regression analysis. When the 95% confidence interval (CI) of the relative risk of a given factor does not include 1, the value is significant (p < 0.05) [31]. Variables that were significant at the p < 0.05 level according to the likelihood ratio test were considered to be risk factors for PRV field strain seropositivity. The strength of the association between a variable and PR prevalence was presented in terms of the odds ratio (OR; a measure of effect size describing the strength of association or non-independence between two binary data values) [17].
Statistical analyses were performed with the SPSS 20.0 software package (SPSS, USA). GraphPad Prism 6.01 (GraphPad Software, USA) was used to create boxplots.
Serological results are presented in Table 2. The data indicated that the seroprevalences of serum samples and herds were 25.0% (1509/6035) and 56.7% (127/224), respectively. The seroprevalence of serum samples from different sized herds in different years showed a tendency toward 2012 > 2013 > 2014. Herds with 50‒100 breeding sows showed higher herd and serum sample seroprevalences than larger herds (> 100 breeding sows).
Based on their economic level and convention, 17 cities of Shandong province can be divided into five regions (Fig. 1). Both sample seroprevalence (panel A in Fig. 2) and herd seroprevalence (panel B in Fig. 2) varied with herd geographical location, and they showed a similar tendency toward west > north > central > south > east (Fig. 2). Among regions, the seroprevalence of 50‒100 sow herds, as well as the seroprevalence of samples collected from such herds, was highest in the north, while the seroprevalence of larger herds, as well as the seroprevalence of samples collected from such herds, was highest in the west (panels A and B in Fig. 2). The average sample seroprevalence of fattening pigs was 28.4% (361/1272). Among the five regions, the sample seroprevalence of fattening pigs in the west was highest, while that from the east was lowest, with rates of 51.6% (96/186) and 16.9% (68/403), respectively. The sample seroprevalence of fattening pigs from the other four regions varied with herd size, except for the central region, and smaller herds had higher sample seroprevalence (panel A in Fig. 3). The average seroprevalence of gilts was 20.9% (403/1929). Among the five regions, the sample seroprevalence of gilts was highest in the south and lowest in the east, with rates of 29.0% (60/207) and 10.8% (54/499), respectively (panel B in Fig. 3). The sample seroprevalence of multiparous sows was 26.3% (745/2834). Among the five regions, the sample seroprevalence of multiparous sows was higher in the west and north than in the other three regions (panel C in Fig. 3).
The results of the univariate analysis are shown in Table 3. When compared with the east, the west (OR, 5.511; 95% CI, 2.141‒14.182) and north (OR, 3.391; 95% CI, 1.595‒7.209) were at higher risk for PRV herd seropositivity (p = 0.001). The 50‒100-sow herds were at higher risk for PRV herd seropositivity than the 101‒300-sow herds (OR, 0.444; 95% CI, 0.230‒0.855) and 301‒500-sow herds (OR, 0.481; 95% CI, 0.231‒1.001; p = 0.039). The PRV infectious risk was significantly higher in herds that purchased gilts of higher body weight, especially those that purchased gilts over 80 kg (OR, 3.195; 95% CI, 1.504‒6.788; p = 0.007). Herds that did not implement all-in/all-out practice exhibited a significant association with PRV herd seropositivity (p = 0.012).
The final multivariable logistic regression analysis for PRV herd seropositivity is summarized in Table 4. Upon multivariate analysis, region (OR, 7.591; 95% CI, 1.971‒16.762; p = 0.021), herd scale (OR, 4.152; 95% CI, 1.852‒13.991; p = 0.002), weight of purchased gilts (OR, 7.003; 95% CI, 1.731‒22.179; p = 0.029) and all-in/all-out practice (OR, 3.594; 95% CI, 1.249‒9.113; p = 0.013) were significantly associated with PRV herd seropositivity.
To date, few studies of the seroprevalence of antibodies against field strains of PRV have been conducted. Moreover, few studies have been investigated to analyze the relationship between risk factors and PR epidemics in China. This study is the first to examine PRV seroprevalence and the associated risk factors in small- and medium-sized farrow-to-finish pig herds in China.
Based on attenuated live marker vaccines and an accompanying specific ELISA, it is easy to effectively control and eradicate PR in pig herds that implement strict biological safety measures. Germany, Sweden, Denmark, and the United Kingdom have eradicated PR, while others, including the United States, France, Poland, and other countries, have eradicated PR from their domestic pig populations [1019]. In China, the PR eradication program has been conducted for several years, and most of the eradicated herds greatly benefited from PRV-free pigs, as evidenced by a decrease in piglet mortality rate and an increase in the annual number of weaned pigs per sow and farrowing number. Undoubtedly, the economic performance of the eradicated herds improved remarkably. However, beginning in late 2011, an epidemic that mostly affected piglets and growing pigs rapidly spread throughout northern China, with a reported mortality rate of 10–50%, while a later epidemic in southern China resulted in relatively slight losses and seroconversion among pigs in eradicated farms. As shown in Table 1, the seroprevalence of serum samples and herds in this study were 25.0% and 56.7%, respectively, and the sample seroprevalence was significantly higher than a previously reported seroprevalence of 11.7% (unpublished data). These results indicate that the outbreak of PRV variant induced a high level of PRV field infections, resulting in seroconversion in many PR-eradicated herds despite vaccination. The seroprevalence of serum samples from different sized herds in different years showed a tendency toward 2012 > 2013 > 2014. These findings indicate that the corresponding comprehensive measures adopted to control PR have played an effective role in Shandong province since 2012. The 50‒100-sow herds showed higher herd and serum sample seroprevalences than larger herds. This was probably due to imperfections in vaccination, management procedures, and biosecurity measures in small-scale herds when compared with larger herds, which lead to a higher risk of PR incidence and epidemics.
As described above, both sample and herd seroprevalences in different regions varied significantly depending on geographical location, and they showed a similar tendency toward west > north > central > south > east. Among the different regions, the seroprevalence of 50‒100 sow herds, as well as the seroprevalence of samples collected from such herds, was highest in the north, while the seroprevalence of larger herds, as well as the seroprevalence of samples collected from such herds, was highest in the west. As a large pig production province, Shandong provided 57,942,000 finishing pigs for slaughter each year, amounting to 10% of the annual total slaughtered finishing pigs in China (data from 2012). Based on the geographical location and significant differences in economic development level, the development of pig production in the five regions is unbalanced. The pig industry in the economically developed east region started early, and it has since exhibited strong consumption demand. As a result, there has been great investment of capital, which has strongly promoted the development of an intensive and large-scale pig industry that exhibits improved productivity. In contrast, the pig industry in the central and south regions started later, and were mainly characterized by moderate-sized breeding herds. The pig breeding levels in the central and south regions that have developed transportation networks are slightly lower than those in the east. As traditional cattle and sheep production regions, the economically less developed west and north regions began pig production later, and at a smaller scale and lower technological level. The subsequent univariate analysis indicated that the west (OR, 5.511; 95% CI, 2.141‒14.182) and north (OR, 3.391; 95% CI, 1.595‒7.209) were at higher risk for PRV herd seropositivity (p = 0.001) than the east. The final multivariable logistic regression analysis showed that region (OR, 7.591; 95% CI, 1.971‒16.762; p = 0.021) was significantly associated with PRV herd seropositivity.
Medveczky and Lomniczi [15] pointed out that the probability of reintroduction of PRV infection in PRV-free herds was positively correlated with increasing herd size. Additionally, Boelaert et al. [2] and Tamba et al. [25] found a positive correlation between breeding herd size and herd seropositivity. Our study has shown that herd size was associated with PRV infection. Herds of 50‒100 breeding sows were at higher risk for PRV herd seropositivity than 101‒300-sow herds (OR, 0.444; 95% CI, 0.230‒0.855) and 301‒500-sow herds (OR, 0.481; 95% CI, 0.231‒1.001; p = 0.039). According to our study, some factors, such as high stocking density, lack of regional breeding management measures for different production phases, and inefficient biosecurity measures in small-scale herds, may lead to a higher risk of infection when compared to better-managed, larger scale herds.
We found no significant association between purchase policy and herd seroprevalence (p = 0.436), although animal purchase is usually considered to be an important infection source [52027]. This finding is in contrast with those of several previous studies. The purchase of infectious pigs was reported to be the most important source of PRV introduction [12], while others reported a higher infection risk associated with homebred gilts replacement [23], as the investigated herds purchased gilts only from PRV-eradicated herds. A later study showed no association between purchase policy and PRV within-herd seroprevalence [2]. This is probably because other factors, such as regional breeding management measures for different production phases, management procedures, stocking density, and quarantine of the purchased gilts, masked the effect of PRV introduction by gilts actively shedding virus [218]. However, the univariate analysis revealed no significantly influence of the quarantine of purchased gilts on PRV herd seropositivity (p = 0.609). Interestingly, the bodyweights of purchased replacement gilts significantly influenced PRV herd seropositivity (p = 0.007). The purchased, lighter replacement gilts with higher levels of maternal and/or vaccine antibodies are probably protected from field PRV strains in infected herds [25], which allows a long time for quarantine and vaccination prior to their estrus.
The all-in/all-out practice in growing sections was a protective measure against infection with PRV field strains, and herds that did not implement all-in/all-out practices displayed 2.24-times higher herd seropositivity than those that did (OR, 2.244; 95% CI, 1.180‒4.264; p = 0.012). Other studies have already reported an association between all-in/all-out practices and a decrease in the prevalence or elimination of circulating PRV in herds [428].
Although we have analyzed the risk factors we collected in detail, many other reported relevant risk factors were not involved in the study because of the limitations of our investigation. The high density of pig herds or the short distance between herds in a region was reported positively associated with the seropositivity of herds. Tamba et al. [25] found that if there were at least 10,000 pigs within 6 km, the risk increased. In another publication, the farms within 2.5 km seemed to be a risk factor [21]. There was no doubt that high pig density in a region was considered to be a risk factor for the circulation and transmission of PRV. Furthermore, the presence of lakes or rivers showed positive association with seropositive status of a herd, since fog and higher humidity increased the possibility of airborne virus transmission [22]. Moreover, Boelaert et al. [2] reported that the presence of finishing pigs, quality of vaccination and herd immunity were associated with PRV herd seroprevalence.
In conclusion, region, herd scale, weight of purchased gilts, and all-in/all-out practice were associated with PRV herd seropositivity, and these risk factors were directly or indirectly linked with PRV-infectious status of the small- and medium-sized farrow-to-finish herds. These findings indicated that we are facing a serious situation regarding the prevention and control of PR. Overall, the results of this study could help predict the next epidemic and implement control measures. However, these results should be confirmed by using a larger sample size in a follow-up study.
Figures and Tables
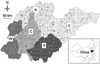 | Fig. 1Map of Shandong and its 17 cities. All of the selected farms where serum samples were collected are marked as black dots (2012), white dots (2013) and squares (2014). The five regions (east, E; west, W; south, S; north, N; central, C) are identified with different patterns. |
 | Fig. 2Seroprevalence of serum samples or herds in different regions classified by different herd sizes. (A) Sample seroprevalence. (B) Herd seroprevalence. Both sample seroprevalence and herd seroprevalence varied with herd geographical location, and they showed a similar tendency toward west > north > central > south > east. Among the different regions, the seroprevalence of 50‒100-sow herds, as well as the seroprevalence of samples collected from such herds, was highest in the north, while the seroprevalence of larger herds, as well as the seroprevalence of samples collected from such herds, was highest in the west. |
 | Fig. 3Sample seroprevalence of different production phases in the five regions. (A) Sample seroprevalence of fattening pigs. (B) Sample seroprevalence of gilts. (C) Sample seroprevalence of multiparous sows. |
Table 2
Seroprevalence (seropositive/total analyzed) of serum samples and herds of PRV of different sized herds from January 2012 to August 2014

Table 3
Risk factors for PRV herd seropositivity expressed as odds ratio (OR) and 95% confidence interval (CI)

*The number of PRV-positive herds. †The number of PRV-negative herds. ‡Herd seroprevalence (PRV-positive herds). §The OR is a measure of effect size, describing the strength of association or non-independence between two binary data values. ∥Variable offered to the subsequent multivariable logistic regression analysis (p < 0.1). ref., references.
Acknowledgments
This project was partially funded by the Agricultural Big Data Foundation of Shandong Agricultural University, China. We appreciate the help of the Swine Disease Laboratory of the Shandong Academy of Agricultural Sciences, the Testing Lab of the Shandong New Hope Liuhe Limited Liability Company, and the Qingdao Yebio Biotechnology Limited Company with data processing and analysis.
References
1. An TQ, Peng JM, Tian ZJ, Zhao HY, Li N, Liu YM, Chen JZ, Leng CL, Sun Y, Chang D, Tong GZ. Pseudorabies virus variant in Bartha-K61-vaccinated pigs, China, 2012. Emerg Infect Dis. 2013; 19:1749–1755.

2. Boelaert F, Deluyker H, Maes D, Godfroid J, Raskin A, Varewijck H, Pensaert M, Nauwynck H, Castryck F, Miry C, Robijns JM, Hoet B, Segers E, Van Vlaenderen I, Robert A, Koenen F. Prevalence of herds with young sows seropositive to pseudorabies (Aujeszky's disease) in northern Belgium. Prev Vet Med. 1999; 41:239–255.

3. Brittle EE, Reynolds AE, Enquist LW. Two modes of pseudorabies virus neuroinvasion and lethality in mice. J Virol. 2004; 78:12951–12963.

4. Deen J, Erickson GA, Scherba G. A retrospective study of factors associated with eliminating circulating pseudorabies virus in sow herds. Swine Health Prod. 1999; 7:147–150.
5. Doyle LP, Gordon AW, Abernethy DA, Stevens K. Bovine tuberculosis in Northern Ireland: risk factors associated with time from post-outbreak test to subsequent herd breakdown. Prev Vet Med. 2014; 116:47–55.

6. Ge X, Wang F, Guo X, Yang H. Porcine circovirus type 2 and its associated diseases in China. Virus Res. 2012; 164:100–106.

7. Gu Z, Dong J, Wang J, Hou C, Sun H, Yang W, Bai J, Jiang P. A novel inactivated gE/gI deleted pseudorabies virus (PRV) vaccine completely protects pigs from an emerged variant PRV challenge. Virus Res. 2015; 195:57–63.

8. Gut M, Jacobs L, Tyborowska J, Szewczyk B, Bienkowska-Szewczyk K. A highly specific and sensitive competitive enzyme-linked immunosorbent assay (ELISA) based on baculovirus expressed pseudorabies virus glycoprotein gE and gI complex. Vet Microbiol. 1999; 69:239–249.

9. Hu D, Zhang Z, Lv L, Xiao Y, Qu Y, Ma H, Niu Y, Wang G, Liu S. Outbreak of variant pseudorabies virus in Bartha-K61-vaccinated piglets in central Shandong Province, China. J Vet Diagn Invest. 2015; 27:600–605.

10. Ketusing N, Reeves A, Portacci K, Yano T, Olea-Popelka F, Keefe T, Salman M. Evaluation of strategies for the eradication of pseudorabies virus (Aujeszky's disease) in commercial swine farms in Chiang-Mai and Lampoon Provinces, Thailand, using a simulation disease spread model. Transbound Emerg Dis. 2014; 61:169–176.

11. Kinker DR, Swenson SL, Wu LL, Zimmerman JJ. Evaluation of serological tests for the detection of pseudorabies gE antibodies during early infection. Vet Microbiol. 1997; 55:99–106.

12. Leontides L, Ewald C, Willeberg P. Herd risk factors for serological evidence of Aujeszky's disease virus infection of breeding sows in northern Germany (1990-1991). Zentralbl Veterinarmed B. 1994; 41:554–560.

13. Lipowski A. Evaluation of efficacy and safety of Aujeszky's disease vaccines. Pol J Vet Sci. 2006; 9:75–79.
14. Luo Y, Li N, Cong X, Wang CH, Du M, Li L, Zhao B, Yuan J, Liu DD, Li S, Li Y, Sun Y, Qiu HJ. Pathogenicity and genomic characterization of a pseudorabies virus variant isolated from Bartha-K61-vaccinated swine population in China. Vet Microbiol. 2014; 174:107–115.

15. Medveczky I, Lomniczi L. Experiences of the eradication of Aujeszky's disease in Hungary between 1976-1991. In : Proceedings of the 14th International Pig Veterinary Society Congress; 7-10 July 1996; Bologna, Italy.
16. Mettenleiter TC. Immunobiology of pseudorabies (Aujeszky's disease). Vet Immunol Immunopathol. 1996; 54:221–229.

17. Monger VR, Stegeman JA, Koop G, Dukpa K, Tenzin T, Loeffen WLA. Seroprevalence and associated risk factors of important pig viral diseases in Bhutan. Prev Vet Med. 2014; 117:222–232.

18. Morrison RB, Marsh WE, Anderson PL, Thawley DG. Factors associated with the seroprevalence of pseudorabies virus in breeding swine from quarantined herds. J Am Vet Med Assoc. 1991; 199:580–583.
19. Müller T, Hahn EC, Tottewitz F, Kramer M, Klupp BG, Mettenleiter TC, Freuling C. Pseudorabies virus in wild swine: a global perspective. Arch Virol. 2011; 156:1691–1705.

20. Rashid MH, Xue C, Islam MT, Islam MR, Cao Y. Risk factors associated with infectious bursal disease in commercial chickens in Bangladesh. Prev Vet Med. 2013; 111:181–185.

21. Rodríguez-Buenfil JC, Alvarez-Fleites M, Alzina-Lopez A, Arjona-Torres MG, Segura-Correa JC, Villegas-Pérez S. Risk factors for Aujeszky's disease in pig herds and detection of field virus antibodies in fattening pigs in the state of Yucatan, Mexico. Prev Vet Med. 2002; 53:205–213.

22. Solymosi N, Reiczigel J, Berke O, Harnos A, Szigeti S, Fodor L, Szigeti G, Boódis K. Spatial risk assessment of herd sero-status of Aujeszky's disease in a county in Hungary. Prev Vet Med. 2004; 65:9–16.

23. Stegeman A, Elbers ARW, Loeffen W, De Jong MCM, Tielen MJM. Rate of successful pseudorabies virus introductions in swine breeding herds in the southern Netherlands that participated in an area-wide vaccination programme. Prev Vet Med. 1996; 27:29–41.

24. Szpara ML, Tafuri YR, Parsons L, Shamim SR, Verstrepen KJ, Legendre M, Enquist LW. A wide extent of inter-strain diversity in virulent and vaccine strains of alphaherpesviruses. PLoS Pathog. 2011; 7:e1002282.

25. Tamba M, Calabrese R, Finelli E, Cordioli P. Risk factors for Aujeszky's-disease seropositivity of swine herds of a region of northern Italy. Prev Vet Med. 2002; 54:203–212.

26. Thrusfield MV. Veterinary Epidemiology. London: Butterworths;1986.
27. Virtanen S, Nikunen S, Korkeala H. Introduction of infected animals to herds is an important route for the spread of Yersinia enterocolitica infection between pig farms. J Food Prot. 2014; 77:116–121.

28. Weigel RM, Austin CC, Siegel AM, Biehl LG, Taft AC. Risk factors associated with the seroprevalence of pseudorabies virus in Illinois swine herds. Prev Vet Med. 1992; 12:1–13.

29. Wu R, Bai C, Sun J, Chang S, Zhang X. Emergence of virulent pseudorabies virus infection in northern China. J Vet Sci. 2013; 14:363–365.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download