Abstract
This study was conducted to establish the feasibility of corneal transplantation using the big-bubble technique (BBT) to perform deep anterior lamellar keratoplasty (DALK) in three dogs. After the cornea was trephined 750 µm, 4 mL of air was injected, and the blanched stroma was removed to expose Descemet's membrane (DM). The donor corneal button, which was gently stripped off the DM, was sutured onto the bare DM of the recipient cornea. The dogs received topical antibiotics every 6 h for 7 days and 2% cyclosporine ointment every 12 h for 1 month. The eyes were examined post-operatively at 7, 14, 21, 28 and 150 days. The central portion of the transplanted cornea stayed transparent while corneal haze developed around the transplanted margin. Menace response was normal even though the transplanted cornea was edematous until 3 weeks after surgery. A marginal haze was rarely observed between the donor and recipient corneas at 150 days after the operation. A spotted haze developed in the central part of the deep stroma near the DM. Upon histopathological examination, the stroma and epithelium of the donor cornea had normal structures. Corneal transplantation using DALK with BBT can be performed in dogs preserving the healthy endothelium.
Corneal ulceration is a common and clinically important ocular disease in dogs. In the case of large and deep corneal defects, various surgical managements have been attempted to promptly and effectively repair the cornea, including conjunctival pedicle graft [25], corneal-scleral transposition [24] and autogenous corneal graft [8]. In addition, preserved biological membranes including pericardium [2], intestinal submucosa [11], amniotic membrane [7] and renal capsule [3] are used in medicine and in veterinary general surgery for deep corneal defects and perforations. However, these methods for treatment of large corneal defects are not able to recover corneal transparency. Additionally, the indicated cases are restricted to certain types of techniques.
Transplantation of various corneal parts is an essential technique for the treatment of severe corneal damage in humans [6] that has been performed successfully in horses for therapeutic and tectonic reasons [9]. Furthermore, penetrating keratoplasty (PK), has been performed in the rabbit cornea in experimental models [22]. However, corneal transplantation is very limited in dogs because of insufficient donor corneas and frequent graft rejections characterized by corneal vascularization, graft failure, and subsequent corneal edema. Therefore, few studies have investigated canine corneal transplantation [20].
Deep anterior lamellar keratoplasty (DALK) removes and replaces the pathologic corneal stroma while preserving the host endothelium, which eliminates the risk of endothelial graft rejection and has a reduced effect on the endothelial cell count [14]. Thus, DALK is indicated for patients with a healthy endothelium to achieve a high success rate for corneal transplantation as an alternative procedure to PK [14]. Several surgical methods were used to bare the Descemet's membrane (DM) during DALK, including layer-by-layer manual dissection [28], hydro-delamination [27], viscoelastic dissection [19] and air injection [4]. The big-bubble technique (BBT) introduced by Anwar and Teichmann is a method that injects air, which forms a large bubble in the stroma to detach the DM during DALK [4]. The present study was conducted to establish the feasibility of corneal transplantation with BBT when performing DALK in dogs.
Three eyes from three healthy male beagles with normal corneas were used in this study. The donor corneas were obtained from dogs scarified in other experiments unrelated to this study. The animal use and experimental protocols were approved by the Institutional Animal Care and Use Committee (SNU-140520-1; Seoul National University, Korea).
Complete ophthalmic examinations were performed before the experiment with a rebound tonometer (TonoVet; Tiolat, Finland), Schirmer Tear Test (Schering-Plough Animal Health, USA), slit-lamp biomicroscope (Topcon SL-D7; Topcon, Japan) and indirect ophthalmoscope (Vantage; Keeler Instruments, USA) with a 30-diopter indirect lens (Classic BIO Lens; Volk Optical, USA). None of the beagles had any corneal diseases.
The surgical procedure was performed under general anesthesia with the BBT as described by Anwar and Teichmann [4]. Before surgery, the central corneal thickness (CCT) was measured with an ultrasonic pachymeter (Pachmate DGH 55; DGH Technology, USA). The central axial cornea was trephined 750 µm with an 8 mm diameter Barron radial vacuum trephine (Katena Products, USA) to create an incision of approximately 80% thickness (panel A in Fig. 1). A partial-thickness superficial anterior keratectomy was performed by dissection with a No. 66 lamellar blade (Katena Products) (panel B in Fig. 1). A small amount of air was introduced into the anterior chamber by intracameral injection at the limbus using a 26-G needle. A 30-G needle attached to a 4 mL filled syringe with the tip manually bent to approximately 30 degrees was introduced with its bevel down into the cornea stroma through the trephination groove and advanced to the center of the cornea (panel C in Fig. 1). At this point, air was injected gently, being forced through the posterior stromal lamella along the path of least resistance, causing the DM to separate from the deep stroma (panel D in Fig. 1). A blanched corneal stroma was incised with a 15° slit-knife (Alcon Laboratories, USA) to let the air escape and collapse the bubble (panel A in Fig. 1). A corneal dissector was carefully inserted and advanced into the cleavage plane that was created until its tip approached the opposite trephination groove. Corneal scissors were used to remove the remaining corneal tissue and expose the DM (panel F in Fig. 1). The donor cornea was gently stripped off the DM and endothelium with a cellulose sponge or forceps. The donor cornea was then punched from the endothelial side with an 8.5 mm-diameter Barron punch (Katena) (panel G in Fig. 1). This prepared donor corneal button was initially sutured onto the bare DM with 4 cardinal 10-0 nylon sutures at 3, 6, 9, and 12 clock-hour positions (panels H and I in Fig. 1). There was also a single running suture with 16 to 18 bites using same suture materials (panel J in Fig. 1). At the conclusion of the surgery, gentamicin and triamcinolone were injected subconjunctivally.
After surgery, atropine eye drop (1%, Isopto Atropine; Alcon, Belgium) was applied two times a day for 3 days and levofloxacine eye drop (0.5%, Cravit; Santen, Japan) was administered every 6 h for 7 days. Cyclosporine ointment (2 mg/g, Optimmune; Schering-Plough, France) was administered every 12 h until 30 days post operatively. All corneal sutures were removed under the topical anesthesia with proparacaine hydrochloride (0.5%, Alcaine; Alcon, New Zealand) at day 21 post-surgery.
The eyes were examined by slit-lamp biomicroscopy (SL-D7), tonometry (TonoVet), and indirect ophthalmoscopy (Vantage) to evaluate corneal condition and graft rejection at 7, 14, 21, 28 and 150 days post-surgery. During these periods, menace response, dazzle reflex, and pupillary light reflex (PLR) were evaluated, and intraocular pressure (IOP) and CCT were measured. In addition, blepharospasm, corneal edema, corneal vascularization, and haze development at the central cornea and suture line were examined clinically. The level of corneal haze was evaluated using the following previously reported clinical grading system [12]: grade 0, completely clear cornea; grade 0.5, trace amount of haze observed with careful oblique illumination; grade 1, mild obscuration of the iris details; grade 2, more prominent haze not interfering with the visibility of fine iris details; grade 3, moderate obscuration of the iris and lens; and grade 4, complete opacification.
At the same time, the CCT was measured using the ultrasonic pachymeter (Pachmate DGH 55; DGH Technology).
The dogs were euthanized at day 150 after surgery, and the eyes enucleated and fixed in 10% formalin. Sections were stained with hematoxylin and eosin and examined by light microscopy. The results of CCT and IOP are expressed as the mean ± SD. Statistical analyses were performed using SPSS for windows (ver. 20; IBM, USA). One was analysis of variance (ANOVA) with Bonferroni's post-hoc assessment was used to test for significance when comparing the CCT. P values < 0.05 were considered significant.
The mean CCT of the three eyes was 817 ± 23 µm before surgery. The central cornea was trephined to a depth of 750 µm for approximately 80% of the corneal thickness. DALK was performed for the three eyes successfully without any DM tearing. After the surgical procedure, no rejection of the corneal implants was observed in the eyes for five months postoperatively (Table 1). The menace response, dazzle reflex, and PLR were normal in all experimented eyes during this period. In addition, the IOP was in the normal range and did not show any significant changes. The fluorescein dye staining test was positive in two cases at 7 days after surgery, and the blepharospasm was examined in the two cases at the same examination time. Because the corneal ulcer was located near the suture line, the ulcer and blepharospasm were related with the spur of the suture materials. No abnormal responses were observed after suture removal.
A corneal haze was developed from 7 days after surgery at the central transplanted cornea and around the suture line (Table 1). The corneal haze of central corneal part was reduced from 21 days after surgery. However, the corneal haze around the junction of the donor and recipient cornea and suture lines increased at 21 days after surgery and remained throughout the experimental period. The central portion of the transplanted cornea remained transparent while a corneal haze developed around the transplanted margin (Fig. 2). Stromal edema of the donor cornea was present from immediately after surgery until three weeks after surgery in all three beagles (Fig. 3), then began to decrease from four weeks after surgery. The corneal thickness was significantly increased at the day 7, 14, 21, and 28 after surgery compared to the CCT of pre-operative examined values (Table 1). The menace response was normal even though the transplanted corneas were edematous.
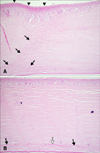
After the sutures were removed, the corneal haze around the implant margin decreased, and the corneal haze near the suture line disappeared. The marginal haze at the junction between the donor and recipient corneas decreased to almost nothing at 150 days after the operation (Fig. 2). At 150 days after surgery, a new spotted haze developed in the central part of the cornea that appeared as a deposited mineral spot. The spotted haze was found in the deep stroma near DM on the slit image (panel F in Fig. 3).
Upon histopathological examination, the stroma and epithelium of the donor cornea had normal structures (Fig. 4). A modified arrangement of the corneal stroma was confirmed in the junction between the donor and recipient corneas. In addition, keratocyte and epithelial proliferation and a distortion in the stromal structure were detected at the junction of the transplant. There were more severe pathological changes in the recipient cornea than in the donor parts. Some particles of the donor's DM in the cornea were observed in the stroma layer (Fig. 4).
The BBT for DALK has been shown to be a valuable method as an alternative to PK with the purpose of reshaping the cornea profile in human patients with keratoconus by removing and replacing most of the corneal ectasia [13]. Thus, DALK is performed without substituting the host's healthy endothelium, and it could reduce the risk of immunological rejection [13]. In addition, DALK has been performed experimentally on rabbits [27] and horses [18]. In this study, BBT with DALK was performed on the canine cornea to establish the feasibility of using this technique in dogs. The shape and transparency of the transplanted cornea were successfully maintained, and 5 months after the surgery there still was no rejection in any of the experimental eyes.
Corneal grafts showed a higher success rate than other organ transplants. To the best of the authors' knowledge, there have been no reports of BBT in dogs to date. BBT, which is normally conducted for human ophthalmology, has not been a feasible procedure in canine eyes [16]. However, according to our results, BBT is not necessarily impossible, although it appears to require a skilled surgeon who knows the technique. A small portion of the stromal layer of the recipient cornea after BBT remained in our experiments, similar to a report by Leiva et al. [16]. However, when the thickness of the remaining stroma in our experiment was estimated by histopathological examination, its thickness was relatively lower in this study compared to the previous report. Similar to our results, very small parts of the stroma remained, which caused the development of corneal haze in studies of human ophthalmology [21].
In addition, part of DM was left in the stromal layer, which determines the junction between the recipient cornea and the donor cornea. Part of the membrane may remain during the process of removing DM from the donor cornea. DM incarceration was reported in human ophthalmology under microscopic examination [15]. The stromal structures of the human cornea after air injection appear similar to those of the dog cornea [5]. Therefore, the results of human ophthalmology support our speculation about the incarcerated part of donor DM.
In our results, the swelling of the stroma of the transplanted cornea was confirmed during the first three weeks after surgery. Edema did not progress and normalized three weeks after surgery. In clinical examinations, an eye that has rejection after corneal transplantation shows conjunctival hyperemia, anterior chamber reaction, keratic precipitates, and graft edema [22]. Corneal transplant rejection is a process in which a corneal graft that has been clear for 5 to 7 days in horses [10] or 2 weeks in humans suddenly develops graft edema in conjunction with signs of anterior segment inflammation [23]. Corneal edema could be produced as part of a sudden rejection of the transplant. However, in this study, corneal edema was present in all of the transplanted corneal parts from the first examination immediately after surgery. Thus, we believe that the corneal edema developed during the surgical procedure, especially in the procedure for donor cornea preparation. When another examination was conducted three weeks after surgery, the corneal edema had resolved itself in all of the cases.
A corneal haze appeared near the junction of the transplant and the suture lines, and overgrowth of the epithelium was observed in these areas. In particular, these reactions were severe on the recipient side of the cornea, but the epithelial cells of the central part of the donor cornea maintained their normal morphology. Overgrown epithelial cells were also more detected on the inside of the recipient cornea. Corneal epithelial breach can be caused by exposed suture knots and a loose suture, which are some of the predictors for the occurrence of corneal haze during the post-operative period [27]. These factors give rise to irritation, leading to a subsequent corneal ulcer and corneal vascularization. Therefore, these reactions could be predisposing factors to graft rejection [27]. In these cases, the haze may invade the adjacent host cornea, which is closer to the vascularization [13].
Much equipment has been developed for human ophthalmology to enable delicate surgical techniques, such as excimer lasers and femtosecond lasers [117]. This equipment has been used to reduce corneal haze, as well as other post-surgical complications. In the veterinary field, BBT can be used to maintain the vision of patients that have a wide range of corneal diseases while preserving the healthy endothelium. Furthermore, considering the quality of life of the animal, BBT is a better surgical method for restoring vision.
In conclusion, corneal transplantation using DALK with the BBT could be performed in a dog that has a large corneal defect and vision loss with a healthy endothelium.
Figures and Tables
Fig. 1
Procedures for deep anterior lamellar keratoplasty using the big bubble technique. (A) The center of the cornea was trephined 750 µm using an 8 mm vacuum trephine. (B) A partial-thickness superficial anterior keratectomy was performed. (C) A 30-gauge needle attached to a 5 cc syringe was inserted at the base of the trephination gutter into the corneal stroma. (D) 4 mL of air were gently injected, causing Descemet's membrane (DM) to separate from the deep stroma. Intrastromal blanching was observed during this procedure. (E) A blanched stroma was incised with a 15° slit-knife to allow air to escape and collapse the bubble for stroma removal. (F) DM was exposed after excising the remaining stroma using corneal scissors. (G) After DM and endothelium were stripped off, the donor cornea was punched from the endothelial side using an 8.5 mm diameter Barron punch. (H) This prepared donor corneal button was fitted onto the exposed Descemet's plane of the recipient cornea. (I) Four cardinal sutures were used with 10-0 nylon at the 3, 6, 9, and 12 clock-hour positions. (J) A single running suture was performed with 16 to 18 bites using the same suture materials.

Fig. 2
Slit-lamp biomicroscopy with direct diffuse illumination of the corneas under 10× magnification. (A) 12 h after surgery, (B) 7 days, (C) 14 days, (D) 21 days, (E) 28 days and (F) 150 days after surgery.
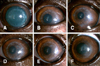
Fig. 3
Slit-lamp biomicroscopy with direct narrow slit (1 mm) under 10× magnification for the stroma. (A) 12 h after surgery, (B) 7 days, (C) 14 days, (D) 21 days, (E) 28 days and (F) 150 days after surgery.

Fig. 4
Histopathological evaluation with hematoxylin and eosin staining at 150 days after surgery. (A) The epithelium was overgrown (arrowhead) at the junction of the donor and recipient corneas. In addition, deformed stromal structures were detected at the transplant junction (black arrow). (B) The central corneal part had a normal corneal structure. A small piece of DM was left in the stroma (white arrow).
Acknowledgments
This research was supported by the College of Veterinary Medicine, the Research Institute for Veterinary Science of Seoul National University, Korea.
References
1. Albarez de Toledo J, de la Paz MF. Manual to laser trephination in corneal transplantation: are patients noticing a difference? Br J Ophthalmol. 2014; 98:717–718.

2. Alio JL, Rodriguez AE, Martinez LM. Bovine pericardium membrane (tutopatch) combined with solid platelet-rich plasma for the management of perforated corneal ulcers. Cornea. 2013; 32:619–624.

3. Andrade AL, Laus JL, Figueiredo F, Batista CM. The use of preserved equine renal capsule to repair lamellar corneal lesions in normal dog. Vet Ophthalmol. 1999; 2:79–82.

4. Anwar M, Teichmann KD. Big-bubble technique to bare Descemet's membrane in anterior lamellar keratoplasty. J Cataract Refract Surg. 2002; 28:398–403.

5. Arenas E, Esquenazi S, Anwer M, Terry M. Lamellar corneal transplantation. Surv Ophthalmol. 2012; 57:510–529.

6. Bahar I, Kaiserman I, Srinivasan S, Ya-Ping J, Slomovic AR, Rootman DS. Comparison of three different techniques of corneal transplantation for keratoconus. Am J Ophthalmol. 2008; 146:905–912.e.1.

7. Barros PS, Garcia JA, Laus JL, Ferreira AL, Salles Gomes TL. The use of xenologous amniotic membrane to repair canine corneal perforation created by penetrating keratectomy. Vet Ophthalmol. 1998; 1:119–123.

8. Brightman AH, McLaughlin SA, Brogdon JD. Autogenous lamellar corneal grafting in dogs. J Am Vet Med Assoc. 1989; 195:469–475.
9. Brooks DE. Penetrating keratoplasty, deep lamellar endothelial keratoplasty, and posterior lamellar keratoplasty in the horse. Clin Tech Equine Pract. 2005; 4:37–49.

10. Brooks DE, Plummer CE, Kallberg ME, Barrie KP, Ollivier FJ, Hendrix DV, Baker A, Scotty NC, Utter ME, Blackwood SE, Nunnery CM, Ben-Shlomo G, Gelatt KN. Corneal transplantation for inflammatory keratopathies in the horse: visual outcome in 206 cases (1993–2007). Vet Ophthalmol. 2008; 11:123–133.

11. Bussieres M, Krohne SG, Stiles J, Townsend WM. The use of porcine small intestinal submucosa for the repair of full-thickness corneal defects in dogs, cats and horses. Vet Ophthalmol. 2004; 7:352–359.

12. Fantes FE, Hanna KD, Waring GO 3rd, Pouliquen Y, Thompson KP, Savoldelli M. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol. 1990; 108:665–675.

13. Fontana L, Parente G, Tassinari G. Clinical outcomes after deep anterior lamellar keratoplasty using the big-bubble technique in patients with keratoconus. Am J Ophthalmol. 2007; 143:117–124.

14. Karimian F, Feizi S. Deep anterior lamellar keratoplasty: indications, surgical techniques and complications. Middle East Afr J Ophthalmol. 2010; 17:28–37.
15. Lang GK, Green WR, Maumenee AE. Clinicopathologic studies of keratoplasty eyes obtained post mortem. Am J Ophthalmol. 1986; 101:28–40.

16. Leiva M, Costa D, Naranjo C, Peña MT. Big bubble and intrastromal air injection techniques for performing deep anterior lamellar keratectomy in the dog. In : 2013 ECVO Annual Conference: the Congress of the European College of Veterinary Ophthalmologists; 16-19 May 2013; Barcelona, Spain.
17. Levinger E, Trivzki O, Levinger S, Kremer I. Outcome of "mushroom" pattern femtosecond laser-assisted keratoplasty versus conventional penetrating keratoplasty in patients with keratoconus. Cornea. 2014; 33:481–485.
18. Martins BC, Brooks DE, Plummer CE, Samuelson DA, Mangan BG, Laus JL. Light microscopic evaluation and scanning electron microscopic analysis of horse eyes following deep anterior lamellar keratectomy. Vet Ophthalmol. 2013; 16:Suppl 1. 42–51.

19. Melles GR, Lander F, Rietveld FJ, Remeijer L, Beekhuis WH, Binder PS. A new surgical technique for deep stromal, anterior lamellar keratoplasty. Br J Ophthalmol. 1999; 83:327–333.

20. McEntyre JM. Experimental penetrating keratoplasty in the dog. Arch Ophthalmol. 1968; 80:372–376.

21. McKee HD, Irion LC, Carley FM, Jhanji V, Brahma AK. Dissection plane of the clear margin big-bubble in deep anterior lamellar keratoplasty. Cornea. 2013; 32:e51–e52.

22. Niederer RL, Perumal D, Sherwin T, McGhee CN. Corneal innervation and cellular changes after corneal transplantation: an in vivo confocal microscopy study. Invest Ophthalmol Vis Sci. 2007; 48:621–626.

23. Panda A, Vanathi M, Kumar A, Dash Y, Priya S. Corneal graft rejection. Surv Ophthalmol. 2007; 52:375–396.

24. Parshall CJ Jr. Lamellar corneal-scleral transposition. J Am Anim Hosp Assoc. 1973; 9:270–277.
25. Soontornvipart K, Tuntivanich N, Kecova H, Raušer P. Conjunctival pedicle graft in dogs and cats: a retrospective study of 88 cases. Acta Vet Brno. 2003; 72:63–69.

26. Sugita J, Kondo J. Deep lamellar keratoplasty with complete removal of pathological stroma for vision improvement. Br J Ophthalmol. 1997; 81:184–188.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download