Abstract
This study was conducted to provide normal reference features for canine and feline anal sacs using ultrasound, low-field magnetic resonance imaging (MRI) and radiograph contrast as diagnostic imaging tools. A total of ten clinically normal beagle dogs and eight clinically normally cats were included. General radiography with contrast, ultrasonography and low-field MRI scans were performed. The visualization of anal sacs, which are located at distinct sites in dogs and cats, is possible with a contrast study on radiography. Most surfaces of the anal sacs tissue, occasionally appearing as a hyperechoic thin line, were surrounded by the hypoechoic external sphincter muscle on ultrasonography. The normal anal sac contents of dogs and cats had variable echogenicity. Signals of anal sac contents on low-field MRI varied in cats and dogs, and contrast medium using T1-weighted images enhanced the anal sac walls more obviously than that on ultrasonography. In conclusion, this study provides the normal features of anal sacs from dogs and cats on diagnostic imaging. Further studies including anal sac evaluation are expected to investigate disease conditions.
Most carnivores have a pair of anal sacs, also referred to as paranal sinuses (sinus paranalis) [116]. The anal sacs are located between the internal and external anal sphincter muscles and each sac opens to the lateral margin of the anus through a single duct [110]. The anal sacs and ducts are lined by stratified squamous epithelium and surrounded by fibrous connective tissue in which sebaceous and apocrine glands are embedded (Fig. 1) [11015]. In dogs, the apocrine glands are concentrated in the fundus, and the sebaceous glands are lined by the ductal area of the anal sac [17]. In cats, both glands exist in the anal sac wall [10]. The anal sacs normally contain secretions [6]. The contents of normal canine and feline anal sacs vary highly in gross appearance. The consistency also varies from watery to creamy to thick or pasty and to the presence or absence of solid material [914].
Anal sac diseases are divided into two main conditions: non-neoplastic (anal sac impaction, anal sacculitis and abscess) and neoplastic [5713]. These conditions are more common in dogs than in cats, occurring in up to 2–12% of canine cases [51112]. The exact reasons for non-neoplastic diseases of the anal sacs have not been investigated, but occlusion of the anal sac ducts is currently believed to be the initiating factor [25]. An anal sac apocrine gland adenocarcinoma is an uncommon tumor in dogs and cats [48]. This type of tumor is usually unilateral, not painful and may be quite small (0.2 to 1 cm). Therefore, a careful rectal examination is required [25]. Regardless of size, these tumors are highly malignant in dogs and often metastasize to regional lymph nodes such as the medial iliac lymph nodes [45].
Diagnosis of an anal sac disease is based on clinical signs and a physical examination, including a rectal examination. Clinical signs associated with anal sac diseases include redness and swelling of the anal sac area, tenesmus, discomfort when sitting down, licking or biting the anal area, tail chasing, painful defecation and scooting (dragging the rear end). However, these symptoms are quite atypical and non-specific, particularly in neoplastic and feline cases [38]. Cytology is a useful tool for diagnosing neoplasia, but no correlation between normal dogs and those with a non-neoplastic anal sac disease was found in a prior study [13].
Therefore, more effective and specific tools are needed to diagnose and detect early anal sac diseases. To the best of our knowledge, no study has explored anal sacs of dogs and cats using diagnostic imaging. The authors of this study anticipate application of diagnostic imaging for evaluation of anal sac diseases because modalities such as ultrasonography and MRI are widely used for soft tissue evaluation in veterinary clinics. Therefore, the present study was conducted to provide normal reference features of canine and feline anal sacs using ultrasound, low-field MRI and a radiograph contrast study in diagnostic imaging.
Ten experimental beagle dogs and eight cats with no clinical or behavioral signs related to anal sac diseases were included in this study. All dogs, which were aged 2–6 (4 years on average), were clinically healthy and had complete blood counts (CBC) within the normal range. All cats were client-owned, 2–5 years in age (3 unknown) and without a history of anal sac diseases. Most cats were domestic shorthairs (n = 5), but a Scottish fold (n = 1), Russian blue (n = 1) and Persian mixed (n = 1) cat were also included. All dogs and cats were mature.
A general radiographic and contrast study of the anal sacs was performed on each experimental individual bilaterally. Right lateral and ventrodorsal (VD) views of the pelvic region were obtained from dogs and cats. The anal sacs were evacuated by gentle squeezing before contrast medium injection. Air was used as the negative contrast medium, and 150 mgI/mL iohexol (Omnipaque 300; GE Healthcare, Ireland) was used as the positive contrast medium. The contrast medium was injected by 22–24 G IV catheter into the anal sac through the anal sac duct until the anal sac was full and overflowing.
All dogs and cats were examined in sternum recumbency or standing position and the tail was reflected over the dorsum (Fig. 2). Ultrasound scanning (SA8000; Medison, Korea) with a linear array transducer (5–9 MHz) was utilized as a sonograph. Dorsal images of the anal sacs were obtained by scanning the anal region after routine skin preparation and use of transmission gel.
Low-field MRI was performed with a 0.25 Tesla MRI scanner (VetMR Grande; Esaote, Italy). The anal sacs were scanned in the transverse and dorsal planes using T1 weighted imaging, T2 weighted imaging, fluid attenuated inversion recovery (FLAIR) and enhanced T1 weighted imaging sequences following intravenous administration of gadopentetate dimeglumine (Magnevist; Schering, Germany) at 0.2 mL/kg (0.1 mmol/kg) body weight.
The anal sacs were invisible on plain radiographs in both dogs and cats. However, the contrast study allowed visualization of location, shape, size (depended on capacity) and margin.
The locations of the anal sacs in dogs were slightly different according to position, but they were usually superimposed over the ischial table region on ventrodorsal views (panels A and B in Fig. 3). Unlike dogs, the anal sacs in cats were observed in soft tissue of the back of the pelvis (panels D and E in Fig. 3). On lateral views, the anal sacs of the dogs were observed at the level of the ischial table in soft tissue dorsal to the pelvis, but the anal sacs in cats were located more caudally (panels C and F in Fig. 3).
The anal sac usually appeared round to oval in shape on ventrodorsal and lateral views. The dog anal sacs were more ellipsoidal, and had the major axis in the craniocaudal dimension on ventrodorsal views and in the dorsoventral dimension on lateral views. In contrast, the appearance of the cat anal sacs was close to round in shape.
The sizes of the anal sacs, which depended on their capacity, varied from 0.6 to 1.8 cm in dogs (major axis) and 0.6 cm to 1.3 cm in cats. The size and shape of the anal sacs were usually symmetric for both dogs and cats, and their margins were smooth.
The anal sacs were located on both sides of the rectum on dorsal images (panel A in Fig. 4). Similar to radiography, the dog anal sacs were ellipsoidal, whereas the cat anal sacs were rounder. Most surfaces of the anal sac tissue were surrounded by the hypoechoic external sphincter muscle. The anal sac tissue occasionally appeared as a hyperechoic thin line (panel B in Fig. 4). The normal anal sac contents of dogs and cats varied in echogenicity. Contents generally appeared hypoechoic with diffuse point-like hyperechoic debris, and the contents of cat anal sacs were more often hyperechoic (panel C in Fig. 4).
The anatomic appearance of the anal sacs on low-field MRI was similar to that of ultrasonography in the same section. However, low-field MRI provided more distinct features than ultrasonography, with wide range images of the anal sacs and adjacent structures (panels A and C in Fig. 5).
While anal sac tissue had slightly higher signal intensity than adjacent external sphincter muscle on T1-weighted images, anal sac tissue observed with post contrast T1-weighted images displayed a distinct signal intensity of the inner layer of the external sphincter muscle (panel B in Fig. 5).
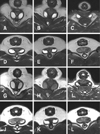
Anal sac contents showed variations in signal intensity and signal pattern because of their diversity. The signal intensity ranges in dogs were from slightly lower to higher than those of muscles on T1-weighted images. The ranges were similar or slightly higher in cats than those in muscles (panels D and E in Fig. 5). On T2-weighted and FLAIR images, the contents of the dog anal sacs showed a high signal intensity. However, a heterogeneous signal was observed in some cats (Fig. 6).
Anal sacs are usually invisible on radiographs because of border effacement with adjacent soft tissue structures. However, gas-containing anal sacs are visible occasionally and may mimic an osteolytic lesion on a ventrodorsal projection. According to a previous study, anal sac gas is seen in 4.9% of females and 6.2% of male dogs [6]. Since that report was based on medium to large breeds, there may be differences in smaller breeds and cats, for which the appearance of gas is generally assumed to be rare to none. Contrast studies of the anal sacs, which can be referred to as sacculography, made it easy to recognize location, size (depended on capacity), shape and margin of the anal sacs.
The anal sacs of cats were more caudally located as a relative anatomical difference observed between the anal sac position and the pelvic bone. The different locations of anal sacs between dogs and cats should be considered when plain radiographs are interpreted.
Despite individual differences, most anal sacs appeared to be symmetric. Consequently, severe alterations in symmetry (location, size, shape and margin) in anal sacs shown by anal sacculography may suggest an abnormality.
Ultrasonographic evaluation of anal sacs is easy, inexpensive and readily accessible. Moreover, the patients show a cooperative attitude during scanning. Thus, ultrasonography is considered the most practical imaging modality for evaluation of anal sacs. Using a high frequency probe and stand-off is recommended to obtain better images because the anal sac is situated superficially.
Previous studies measured anal sac size indirectly or post-mortem, but ultrasonography can be applied to anal sac measurement under natural conditions. The reported diameters of anal sacs normally vary between 0.5 and 4 cm in dogs, while they are approximately 1 cm in cats [1016]. Distension of the anal sac is often used to diagnose disease conditions, but anal sacs filled with contents are not necessarily diseased [7]. According to a previous report, there is no correlation between anal sac size and an animal's weight or age, either for normal or diseased conditions [16].
The ultrasonographic features of anal sac contents are expected vary because of their variable viscosity and consistency. Their consistency may depend on cellularity [9], which can affect their echogenicity. Therefore, it is critical, but difficult to determine the normal ranges of imaging features. Nevertheless, as it is possible to evaluate muscles and other soft tissue structures, ultrasonography may be useful for detection of small masses and invasion into adjacent tissues. In case of hypercalcemia suspected an anal sac neoplasia, scanning by ultrasonography would be an alternative option to diagnose.
The signals of the anal sac contents varied on low-field MRI. In this study, cats occasionally had heterogeneous signals. However, this heterogeneity may have been due to viscosity rather than species specificity. The contents of cat anal sacs tended to have a higher signal than those of dog anal sacs on T1-weighted images. It seems that the lipid secretions from the sebaceous gland represent a high proportion of the anal sac secretions, and this secretion may help prevent obstruction of the anal sac duct [2].
Contrast medium enhanced the anal sac wall more obviously in cats and dogs than ultrasonography or T1 weighted images. Low-field MRI provides an excellent visualization of soft tissues and other adjacent structures and is considered the gold standard for assessing aggressive lesions in soft tissue structures.
If a lesion originates from anal sac tissues that are associated with a wide range, such as pelvic bone, it is expected to be evaluated by CT. However, no such samples were observed in this study. Nevertheless, CT and MRI are not appropriate for the general clinics as they are expensive and require anesthesia.
It should be mentioned that this study had two limitations. Specifically, only one breed of dog and a few breeds of cats were investigated. Additionally, no data pertaining to the pathologic status of anal sacs were provided. Therefore further studies are needed before this method can be applied clinically.
The results of this study provided normal reference features of canine and feline anal sacs by diagnostic imaging. Amongst the investigated methods, ultrasonography is expected to be applied in further anal sac evaluation studies to identify disease conditions, as it is more practical than the other modalities.
Figures and Tables
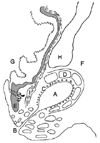 | Fig. 1Diagram of a dorsal section of the anal sac of a dog. (A) Anal sac cavity. (B) Anal sac duct opening. (C) Apocrine glands. (D) External sphincter muscle. (E) Internal sphincter muscle. (F) Fat of rectoischial fossa. (G) Anal canal. (H) Levator ani muscle. (I) Longitudinal muscle layer of rectum [15]. |
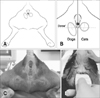 | Fig. 2Positioning for anal sac scan (A and C). Approaching for ultrasonographic examination of the anal sac (B and D). The dog (C) and cat (D) were sedated, but sedation was not essential. |
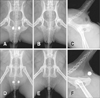 | Fig. 3Ventrodorsal (A, B, D and E) and lateral (C and F) view on radiograph of canine (A–C) and feline (C, D and F) pelvic region. The oval-shaped anal sacs were superimposed over the ischial table region on positive (A) and negative (B and C) contrast study images in dogs. However, the round-shaped anal sacs were observed in the soft tissue of the caudal region of the pelvis on positive (D and F) and negative (E) contrast study images in cats. The anal sac duct was visible as a radiopaque line (F). |
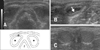 | Fig. 4Dorsal sonographic and diagram (A) of canine anal sac. Dorsal schematic sonograph image (B) of canine anal sac. Anal sac tissue (glands) appears as a hyperechoic thin line (arrow). Dorsal sonographic images of feline anal sac (C). The appearance of the anal sac was round, and their contents appear hypoechoic to similar to external sphincter muscle. Asterisks, anal sac contents; e, external sphincter muscle; r, rectum. |
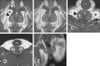 | Fig. 5Dorsal (A, B and E) and transverse (C and D) low-field MRI T1-weighted images of canine (A–C) and feline (D and E) anal sac. Increased signal intensity of anal sac tissue was identified on T1-weighted enhanced images (B). Asterisks, fat of ischiorectal fossa; arrow, levator ani muscle; arrow heads, external sphincter muscle; dagger, anal sac contents; r, rectum. |
Acknowledgments
This study was presented in part at the Annual Meeting of the American College of Veterinary Radiology; October 21-24, 2014; St. Louis, Missouri, USA.
References
1. Al-bagdadi F. The integument. The digestive apparatus and abdomen. In : Evans HE, de Lahunta A, editors. Miller's Anatomy of the Dog. 4th ed. St. Louis: Saunders;2013. p. 75. p. 325.
2. Aronson L. Rectum and anus. In : Slatter DH, editor. Textbook of Small Animal Surgery. 3rd ed. Philadelphia: Saunders;2003. p. 682–708.
3. Bennett PF, DeNicola DB, Bonney P, Glickman NW, Knapp DW. Canine anal sac adenocarcinomas: clinical presentation and response to therapy. J Vet Intern Med. 2002; 16:100–104.

4. Brearley MJ. Epithelial and other solitary skin tumours. In : Dobson JM, Lascelles BDX, editors. BSAVA Manual of Canine and Feline Oncology. 2nd ed. Quedgeley: British Small Animal Veterinary Association;2003. p. 152–160.
5. Craven M. Rectoanal disease. In : Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 7th ed. St. Louis: Elsevier Saunders;2010. p. 1604–1606.
6. Dennis R, Penderis J. Radiology corner—anal sac gas appearing as an osteolytic pelvic lesion. Vet Radiol Ultrasound. 2002; 43:552–553.

8. Elliott JW, Blackwood L. Treatment and outcome of four cats with apocrine gland carcinoma of the anal sac and review of the literature. J Feline Med Surg. 2011; 13:712–717.

9. Frankel JL, Scott DW, Erb HN. Gross and cytological characteristics of normal feline anal-sac secretions. J Feline Med Surg. 2008; 10:319–323.

10. Greer MB, Calhoun ML. Anal sacs of the cat (Felis domesticus). Am J Vet Res. 1966; 27:773–781.
12. Harvey CE. Incidence distribution of anal sac disease in the dog. J Am Anim Hosp Assoc. 1974; 10:573–577.
13. James DJ, Griffin CE, Polissar NL, Neradilek MB. Comparison of anal sac cytological findings and behaviour in clinically normal dogs and those affected with anal sac disease. Vet Dermatol. 2011; 22:80–87.

14. Lake AM, Scott DW, Miller WH Jr, Erb HN. Gross and cytological characteristics of normal canine anal-sac secretions. J Vet Med A Physiol Pathol Clin Med. 2004; 51:249–253.

15. McColl I. The comparative anatomy and pathology of anal glands. Arris and Gale lecture delivered at the Royal College of Surgeons of England on 25th February 1965. Ann R Coll Surg Engl. 1967; 40:36–67.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download