Abstract
Infectious coryza (IC) is an infectious disease caused by Avibacterium (Av.) paragallinarum. IC is known to cause economic losses in the poultry industry via decreased egg production in layers. Between 2012 and 2013, Av. paragallinarum was isolated from seven chicken farms by Chungbuk National University. We identified Av. paragallinarum, the causative pathogen of IC by polymerase chain reaction (PCR) and serovar serotype A, by multiplex PCR. Antibiotic sensitivity tests indicated that a few field-isolated strains showed susceptibility to erythromycin, gentamicin, lincomycin, neomycin, oxytetracycline, spectinomycin, and tylosin. A serological survey was conducted to evaluate the number of flocks that were positive for Av. paragallinarum by utilizing a HI test to determine the existence of serovar A. Serological surveys revealed high positivity rates of 86.4% in 2009, 78.9% in 2010, 70.0% in 2011, and 69.6% in 2012. We also challenged specific pathogen-free chickens with isolated domestic strains, ADL121286 and ADL121500, according to the measured efficacy of the commercial IC vaccine, PoulShot Coryza. We confirmed the effectiveness of the vaccine based on relief of clinical signs and a decreased re-isolation rate of ADL121500 strain. Our results indicate IC is currently prevalent in Korea, and that the commercial vaccine is effective at protecting against field strains.
Infectious coryza (IC) is an acute respiratory disease in chickens caused by the pathogen Avibacterium (Av.) paragallinarum, once known as Haemophilus paragallinarum. Taxonomic differences resulted in its designation as Av. paragallinarum after phenotypic and genotypic investigation [5]. IC is a typical economic disease resulting in poor growth performance in broilers and a decline in egg production in the layer. Major clinical signs include serous nasal discharge in the upper respiratory track and edema of the face and wattle in chickens, regardless of age [7]. However, exhibition of clinical signs can vary depending on age and breed, and the duration and severity of these signs can be affected by factors such as poor housing, parasitism, inadequate nutrition, and mixed infection from other infectious diseases such as fowlpox, infectious bronchitis, laryngotracheitis, Mycoplasma gallisepticum, and pasteurellosis [4722].
Page [19] recognized three serovars of Av. paragallinarum, A, B, and C, which can be distinguished by hemagglutination inhibition (HI) tests using chicken erythrocytes fixed with glutaraldehyde [1318]. Another classification method, the Kume scheme, classifies Av. paragallinarum into nine serovars (A-1, A-2, A-3, A-4, B-1, C-1, C-2, C-3, and C-4). This method is also based on HI tests, but the scheme is only used in a few select laboratories because it is technically demanding to perform [215].
Av. paragallinarum has been isolated worldwide, but causes the greatest economic losses to the poultry industry of developing countries because of the presence of many variable pathogens or stress factors associated with poor environmental conditions for growth. Outbreaks of IC have been reported in Japan, China, and Taiwan, and the isolates were classified as A or C by the Page scheme serovar [101426]. Page scheme serovar A was reported in Korea in the 1980s [17]. Because of a lack of studies since the first IC report, a report regarding Page scheme serovar B and C has not yet been published.
Diagnostic methods for IC include direct isolation, the HI test for serovar A, and polymerase chain reaction (PCR). For direct isolation, the pathogen can be isolated from sterile cotton swabs obtained from the infraorbital sinus, trachea, and air sac. However, the pathogen must be isolated during the acute stage of infection after 1 to 7 days of incubation, which complicates direct isolation [7]. The HI test, which is one of the most widely used serological test, is often utilized to detect changes in antibody titers in cases of field infection or vaccination, and is useful for evaluating the prevalence of IC in certain areas or conducting retrospective/epidemiological studies [3]. Chen et al. [9] employed PCR to diagnosis Av. paragallinarum and concluded that this method is swift and accurate. Subsequent development of enterobacterial repetitive intergenic consensus PCR has supplemented the Page serovar classification method, but this analysis may not be straightforward [25]. Recent reports have utilized multiplex PCR and PCR restriction fragment length polymorphism as a serotyping method for Page serovars A, B, and C by targeting the outer membrane protein and main defense antigen HMTp210 genes of Av. paragallinarum [21]. These genetic analyses in addition to the classic IC diagnostic methods of bacterial isolation and serological tests for serovar A are useful for diagnosis and identification of IC in field cases or laboratory experiments.
The present study was conducted to examine the prevalence of IC and further characterize field isolates of Av. paragallinarum because no studies of IC have been conducted in Korea since the 1980s. Moreover, tests of the efficacy of the only commercially vaccine available in Korea at present were conducted to evaluate protection against field infection of Av. paragallinarum.
Av. paragallinarum cultured on chocolate agar was incubated in brain heart infusion (BHI) broth (BD, USA) with 5% (v/v) heat-inactivated swine serum (Sigma-Aldrich, USA) and 0.01% nicotinamide adenine dinucleotide (Sigma-Aldrich) at 37℃ for 16 h [14]. The enriched broth was centrifuged at 4,000 × g for 20 min, then washed 3 times with phosphate buffer solution (PBS) with 0.01% thimerosal (Sigma-Aldrich) and incubated at 4℃ overnight. The hemagglutination test was as follows. PBS (25 µL per well) was added to a 96 well microtiter plate, followed by 25 µL of antigen, and then diluted two-fold. A 25 µL aliquot of fresh chicken RBC made from Alsever's solution was added to each well, after which plates were incubated at room temperature for 40 min after mixing. The concentration of the antigen was diluted to 4 hemagglutinating units (HAU) for the HI test for serovar A.
The HI test for serovar A was performed with a 4 HAU antigen concentration from field strains and antibody of Page serovar A (221 strain). Positive control serum of serovar A (221 strain) for the HI test was generously supplied by ChoongAng Vaccine Laboratories (Korea), and serum from specific pathogen-free (SPF) chickens was used as the negative control. Twenty five µL of positive serum (221 strain) and negative serum were added to a 96-well plate and diluted two-fold with PBS. An equal volume of serum with an antigen concentration of 4 HAU was added to each well, followed by 45 min incubation at room temperature for 20 min after mixing [14]. Next, 25 µL of fresh chicken RBC produced from Alsever's solution were added to each well, after which the plate was incubated at room temperature for 40 min. Among serum samples submitted to the Chungbuk National University Avian Disease Laboratory from 2009 to 2012, those obtained from flocks with a history of respiratory distress or decreased egg production were chosen. Flocks with one or more positive results from the five serum samples were considered positive.
To determine if the isolated pathogen was Av. paragallinarum, all isolated field strains were subjected to HP-2 PCR [89]. Viral Gene-spin (iNtRON Biotechnology, Korea) was used for DNA extraction. The PCR mixture consisted of Maxime PCR PreMix (iNtRON Biotechnology), 1 µL of each forward and reverse primer, 1 µL of extracted template gene, and 17 µL of distilled water. The primer used was the N1/R1 primer set for HP-2 PCR described by Chen et al. [8]. The PCR protocol consisted of an initial step at 94℃ for 2 min followed by 40 cycles of 94℃ for 20 sec, 65℃ for 10 sec and 72℃ for 40 sec and then a final step at 72℃ for 2 min. The PCR products were examined by agarose gel electrophoresis, with the band at 0.5 kbp signifying Av. paragallinarum [8]. After confirming the identity of the pathogen as Av. paragallinarum, multiplex PCR was conducted to distinguish between Page serovar A, B, and C. The primer set used to distinguish regions of the outer membrane protein HMTp210 was used to confirm band sizes. Multiplex PCR mixture consisted of Maxime PCR PreMix, 1 µL of each A, B, and C serotype forward and reverse primer sets, 1 µL of template, and 13 µL of distilled water. The PCR protocol consisted of an initial step at 94℃ for 2 min followed by 30 cycles of 94℃ for 20 sec, 56℃ for 12 sec and 72℃ for 60 sec, and then a final step at 72℃ for 3 min. The PCR products were examined by agarose gel electrophoresis. Bands at 0.8, 1.1, and 1.6 kbp correspond to Page scheme serovars A, B, and C, respectively [21].
The disk diffusion method was used to test antibiotic sensitivity in field isolates of Av. paragallinarum. The disk diffusion test was performed as recommended by the Clinical and Laboratory Standards Institute [12], with some modification by Chukiatsiri et al. [11]. The recommended reference strain (Escherichia coli ATCC 25922) was used as the quality control strain. Twelve antibiotics were chosen based on those commonly used in farms, ampicillin, ceftiofur, cloxacillin, enrofloxacin, erythromycin, gentamicin, lincomycin, neomycin, oxytetracycline, sulfamethoxazole-trimethoprim, spectinomycin, and tylosin. All of the antimicrobial disks were from Liofilchem (Italy). The isolated Av. paragallinarum was incubated at 37≧ for 16 h in BHI broth with 5% (v/v) heat-inactivated swine serum and 0.01% nicotinamide adenine dinucleotide. The enriched medium was spread on modified TM/SN agar plates at a concentration of 108 CFU/mL. Antibiotic disks were dispensed on the plates and incubated at 37℃ for 24 h. Diameters of the regions where growth was inhibited were measured with Vernier calipers in millimeters [6]. These measurements were grouped into categories of sensitive, intermediate, or resistant based on the values in Table 1.
Chicks were hatched and grown from SPF eggs (Namduk SPF, Korea) until postnatal 40 days old under identical conditions in separate sterile isolators (Three-Shine, Korea). At 40 days of age, 55 chickens were randomly divided into five groups of 11 subjects. The details describing the groups are listed in Table 2, with two vaccinated groups (group A and C), two non-vaccinated groups (group B and D), and a negative control group (group E). Group A and C were administered 1 mL intramuscular injections of the vaccine PoulShot Coryza (ChoongAng Vaccine Laboratories) at 40 days of age. Among of isolated field strains, ADL121286 and ADL121500 were selected for challenge. Because the ADL121286 field strain caused mild clinical signs and there were no secondary infections. Field strain ADL121500 produced severe clinical signs and infectious bronchitis. At 54 days of age, each group was challenged by nasal inoculations of 0.2 mL. Specifically, group A and B were inoculated with ADL121286, group C and D were inoculated with ADL121500, and group E was inoculated with medium. The concentrations of the inoculated field strains were adjusted to 1 × 108 CFU/mL and the actual count was confirmed by spread plating of 10 fold dilutions. After inoculation, three, three, and five chickens from each group were euthanatized at 5 days post challenge (dpc), 10 dpc, and 15 dpc, respectively. If Av. paragallinarum was re-isolated from either infraorbital sinus, the animal was considered positive for infection. Samples were also collected from both infraorbital sinuses and the choanal cleft with cotton swabs and diluted in 1 mL PBS, after which the diluted mixture was used for HP-2 PCR. If more than one of the three PCR samples of each chickens was positive the animal was considered positive for infection. Additionally, HI mean titer and HI titer ranges were determined via HI tests for serovar A, which were carried out using chicken serum obtained from all chickens at pre-vaccinated 40 days of age and pre-challenged 54 days of age, as well as from the chicken serum of euthanatized animals at 5 dpc, 10 dpc, and 15 dpc. The HP211 strain was used as the antigen in these HI tests for serovar A. Throughout the experiment, organisms were grown in a high efficiency particulate air filtered isolate system maintained at room temperature. Antibiotics-free feed was provided by NongHyup feed (Seoul, Korea) and water was provided by Laboratory Animal Research Center (Chungbuk, Korea). All processes were conducted in accordance with the guidelines of the Institute of Laboratory Animal Resources (confirmation No. CBNUA-637-13-01).
Significant differences between serological surveys based on the year the survey was conducted and the age of the animals were identified by the chi-square test and Fisher's exact test. To compare the clinical sign scores of experimental groups, one-way ANOVA was used to compare the means of each group and the Bonferroni correction was employed for multiple comparisons. A p < 0.05 was considered to indicate statistical significance for all analyses.
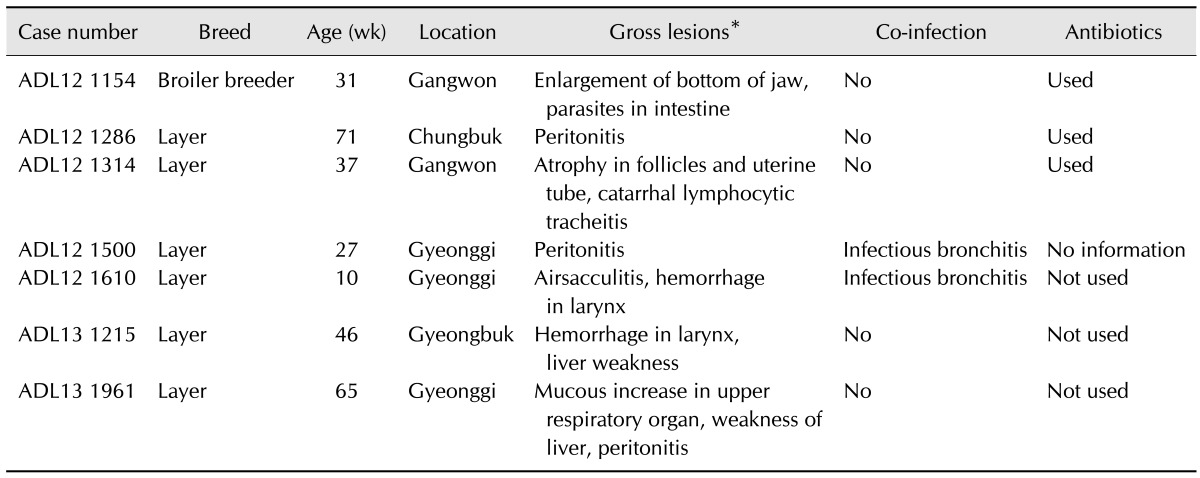
As depicted in Table 3, attempts to isolate Av. paragallinarum have been conducted by swabbing the infraorbital sinus of the chickens submitted from poultry farms with a history of decreased egg production to the Avian Disease Laboratory, Chungbuk National University. Common observations from the submitted cases include a swelling of the infraorbital sinus, edema of the surrounding tissues, sometimes extending to the wattles, and hyperemia of the conjunctiva. Edema in the margins of eyelids and wattle varied from severe to mild.
Field strain of ADL121154, ADL121286, ADL121314, ADL121500, ADL121610, ADL131215, and ADL131961 were identified by the presence of clear dew-like colonies in chocolate agar. In HP-2 PCR, seven field strains and positive control strain 221 were also confirmed as Page serovar A based on the presence of bands at 0.5 kbp. The strains were also confirmed as Page serovar A by multiplex PCR based on the presence of bands at 0.8 kbp.
We evaluated the susceptibility of the seven field strains of Av. paragallinarum to 12 antibiotics, as described in Table 1. The strains were shown to be the lowest susceptible against enrofloxacin (57.1%). The strains were relatively more susceptible against ampicillin (71.4%), cloxacillin (85.7%), gentamicin (85.7%) and sulfamethoxazole-trimethoprim (85.7%). The strains were shown to be sensitive against erythromycin, gentamicin, lincomycin, neomycin, oxytetracycline, spectinomycin, and tylosin.
The results of the serological survey are summarized in Table 4 according to tested year and age. Groups were divided by age, with the first group consisting of birds prior to egg laying age (1–19 weeks). Groups were further divided into separate age groups about 10 weeks apart for each group. The positivity rate of the 1–19 weeks group was 33.3%, which was relatively lower than that of other groups. The positivity rate of groups above 20 weeks of age ranged from 70.4% to 88.2% with no significant difference among groups. Overall positivity was highest in 2009 (86.4%) and lowest in 2012 (69.6%), but the differences between years were not significant.
Average clinical sign score: The clinical sign scores for challenged groups are depicted in Table 5. The average clinical sign scores for groups challenged with ADL121286 were 0.38 ± 0.23 for group A and 0.24 ± 0.14 for group B. There was no statistical difference among groups. However, the average clinical sign score of groups challenged with ADL121500 strain was 0.80 ± 0.13 for group C and 1.34 ± 0.41 for group D, with vaccinated group C exhibiting significantly lower clinical sign scores. No clinical signs were observed in negative control group E.
Re-isolation rate: Re-isolation rates based on media and PCR from samples obtained from animals euthanized on 5 dpc, 10 dpc, and 15 dpc are depicted in Table 5. Re-isolation was conducted by culture in media and confirmed by PCR. While re-isolation rate was relatively low in group A (33%) in media, it was shown to be 100% by PCR at 5 dpc. The re-isolation rates of groups B, C and D were shown to be 100% in both media and PCR at 5 dpc. Re-isolation rates decreased in both media and PCR in groups A, B, and C at 10 dpc. However, the re-isolation rate of group D was relatively high throughout the experiment, showing isolation rates of 100% in media and 80% for PCR, even at 15 dpc. Strains were not re-isolated from vaccinated group A and C at 15 dpc. The re-isolation rate was moderate in group B and relatively high in D group at 15 dpc. Overall, the media and PCR re-isolation rates were shown to be similar. Strains were not isolated from negative control E group by media or PCR.
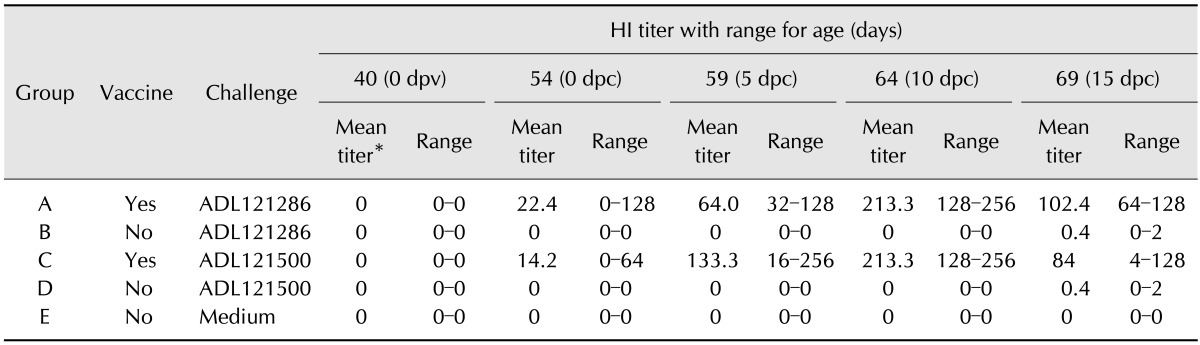
HI test: Chickens were vaccinated at 40 days of age prior to challenge with field strain. Chickens were challenged at 54 days of age and HI titers were obtained at 5 dpc, 10 dpc and 15 dpc after challenge. The titers are displayed in Table 6. Both vaccinated and non-vaccinated groups showed negative titers prior to vaccination at 40 days of age. At 54 days of age in 0 dpc, the mean titers of the vaccine groups were 22.4 and 14.2, respectively, showing positive titers. Both vaccine groups showed positive titers at 54 days of age, with highest being observed at 59 days of age. Both groups showed decreases in titer at 64 days of age. After challenge with field strain, group B and D showed positive titers at 64 days of age, resulting in all groups showing positive titers with the exception of control group E at 64 days of age.
The causative IC agent, Av. paragallinarum, was difficult to isolate as use of antibiotics in feed was allowed before July of 2011 in Korea. However, use of antibiotics in feed was prohibited from July 2011 (Ministry of Agriculture, Food and Rural Affairs). Hence, we attempted to isolate Av. paragallinarum from chickens exhibiting clinical signs of IC such as edema in the face and wattle and drop in egg production. Overall, seven field strains of Av. paragallinarum were isolated, and PCR and multiplex PCR confirmed all seven strains as Page serovar A.
Av. paragallinarum is known to be resistant to streptomycin and other antibiotics [1]. In Taiwan, Av. paragallinarum showed high resistance to antibiotics used against IC, such as streptomycin, sulfonamides, kanamycin, and neomycin, which has been confirmed to be related to multidrug-resistant plasmids [14]. As shown in Table 3, field strains isolated in Korea were resistant to enrofloxacin, ampicillin, ceftiofur, cloxacillin and sulfamethoxazole-trimethoprim. Although the use of antibiotics to treat IC is rare in Korea, this study showed Av. paragallinarum has some resistance against common antibiotics clinically used in the field. These resistant Av. paragallinarum would still remain in the carrier chickens even after treatment with antibiotics, causing irregular recurrences [17]. Thus, it is recommended that antibiotic sensitivity tests be conducted for each case of Av. paragallinarum prior to selection and application of effective antibiotics.
In a serological survey, the rate of cases exhibiting clinical signs in the upper respiratory tract and decrease in egg production were high, with positivity rates of 86.4% in 2009, 78.9% in 2010, 70.0% in 2011, and 69.6% in 2012. Random monitoring of farms in Pakistan revealed a positivity rate of 5.3% in 2010 [23]. Studying the accurate IC positivity in Korea was not feasible in this study because we focused on cases already exhibiting clinical problems or selecting samples obtained from routine serological inspections. Since the survey was focused on flocks with clinical signs in the upper respiratory tract and showing decreased egg production, the IC positivity rate observed in the present study was higher than that of Pakistan. Additionally, due to the absence of sales of IC vaccine supplied by ChoongAng Vaccine Laboratories before 2012, the serological survey result demonstrates the widespread nature of domestic Av. paragallinarum and the degree of constant economic loss caused by the IC agent.
As shown in Table 5, groups challenged with ADL121286 (group A and B) showed no significant difference in clinical signs score, and re-isolation rates were high in both groups at 5 dpc, but gradually decreased at 10 dpc and 15 dpc. However, re-isolation rates in group B at 15 dpc reflect instances of constant infection compared with vaccinated group A, implying a role of the vaccine in decreasing the period of infection. Groups challenged with ADL121500 (group C and D) showed significant differences in clinical sign score, and the re-isolation rate of group C was 0.0% at 15 dpc compared to group D, which showed 100% re-isolation in media and 80% for PCR. Thus, the relief of clinical signs in group C and decreased re-isolation rate confirms that the vaccine provide some protection against strain ADL121500 strain. The clinical sign score of serovar A was observed for a 10 day period by Zhao et al. [28]. Our scoring results for the ADL121286-inoculated group coincided with Zhao's work, as clinical signs reached a peak at 5 dpc and were relieved around day 10. Clinical signs of the ADL121500-challenged group (group D) reached their peak at 7 dpc and had a longer duration. These results indicate that the duration of clinical signs can differ, even for strains within the same serovar. Additionally, protective properties were more clearly expressed in strains with stronger pathogenicity, as vaccinated group C had the briefest period of clinical signs and reduced re-isolation rates.
Yamaguchi et al. [27] reported a positive titer 2 weeks after serovar A vaccination and a complete shift to positivity at 3 weeks, while a positive titer was observed only to some degree after 3 weeks in chickens inoculated with a field strain of serovar A. We found a complete shift to positivity in 2 weeks for vaccinated groups A and C, and boosting was confirmed after field strain inoculation. Additionally, groups B and D showed a low positive titer after challenge inoculation at 15 dpi. These findings show that an appropriate serologic response was present in this study.
This study confirmed the presence of domestically isolated Av. paragallinarum and revealed the antibiotic specificity of the strains against certain antibiotics. We also confirmed domestic IC to cause clinical signs in the upper respiratory tract via serological survey. An available vaccine based on the domestically available Av. paragallinarum serovar A (221 strain) strain was confirmed to be relatively effective against domestically isolated field strains. The protection abilities of the vaccine against the highly pathogenic ADL121500 strain were proven to be statistically significant, whereas the protection against the relatively low pathogenic ADL121286 strain was not confirmed because of a lack of statistical evidence. Additional research and experiments are required to further explore the protection abilities of the vaccine.
References
1. Blackall PJ. Antimicrobial drug resistance and the occurrence of plasmids in Haemophilus paragallinarum. Avian Dis. 1988; 32:742–747. PMID: 3202771.
2. Blackall PJ. Infectious coryza. In : Dufour-Zavala L, editor. American Association of Avian Pathologists. A Laboratory Manual for the Isolation, Identification and Characterization of Avian Pathogens. 5th ed. Athens: American Association of Avian Pathologists;2008. p. 22–26.
3. Blackall PJ. Infectious coryza: overview of the disease and new diagnostic options. Clin Microbiol Rev. 1999; 12:627–632. PMID: 10515906.

4. Blackall PJ. Vaccine against infectious coryza. Worlds Poult Sci J. 1995; 51:17–26.
5. Blackall PJ, Christensen H, Beckenham T, Blackall LL, Bisgaard M. Reclassification of Pasteurella gallinarum, [Haemophilus] paragallinarum, Pasteurella avium and Pasteurella volantium as Avibacterium gallinarum gen. nov., comb. nov., Avibacterium paragallinarum comb. nov., Avibacterium avium comb. nov. and Avibacterium volantium comb. nov. Int J Syst Evol Microbiol. 2005; 55:353–362. PMID: 15653900.
6. Blackall PJ, Reid GG. Further characterization of Haemophilus paragallinarum and Haemophilus avium. Vet Microbiol. 1982; 7:359–367. PMID: 6758314.
7. Blackall PJ, Soriano EV. Infectious coryza and related bacterial infectious. In : Swayne DE, Glisson JR, McDougald LR, Nolan LK, Suarez DL, Nair V, editors. Diseases of Poultry. 13th ed. Ames: Wiley-Blackwell;2013. p. 859–874.
8. Chen X, Miflin JK, Zhang P, Blackall PJ. Development and application of DNA probes and PCR tests for Haemophilus paragallinarum. Avian Dis. 1996; 40:398–407. PMID: 8790892.
9. Chen X, Song C, Gong Y, Blackall PJ. Further studies on the use of a polymerase chain reaction test for the diagnosis of infectious coryza. Avian Pathol. 1998; 27:618–624. PMID: 18484051.

10. Chen X, Zhang P, Blackall PJ, Feng W. Characterization of Haemophilus paragallinarum isolates from China. Avian Dis. 1993; 37:574–576. PMID: 8363520.
11. Chukiatsiri K, Sasipreeyajan J, Blackall PJ, Yuwatanichsampan S, Chansiripornchai N. Serovar identification, antimicrobial sensitivity, and virulence of Avibacterium paragallinarum isolated from chickens in Thailand. Avian Dis. 2012; 56:359–364. PMID: 22856194.
12. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Informational Supplement. NCCLS document M31-S1. Wayne: Clinical and Laboratory Standards Institute;2004.
13. Eaves LE, Rogers DG, Blackall PJ. Comparison of hemagglutinin and agglutinin schemes for the serological classification of Haemophilus paragallinarum and proposal of a new hemagglutinin serovar. J Clin Microbiol. 1989; 27:1510–1513. PMID: 2768440.
14. Hsu YM, Shieh HK, Chen WH, Sun TY, Shiang JH. Antimicrobial susceptibility, plasmid profiles and haemocin activities of Avibacterium paragallinarum strains. Vet Microbiol. 2007; 124:209–218. PMID: 17485180.
15. Kume K, Sawata A, Nakai T, Matsumoto M. Serological classification of Haemophilus paragallinarum with a hemagglutinin system. J Clin Microbiol. 1983; 17:958–964. PMID: 6874914.
16. Matsumoto M, Yamamoto R. A broth bacterin against infectious coryza: immunogenicity of various preparations. Avian Dis. 1971; 15:109–117. PMID: 5547749.

17. Namgoong S, An SH, Kim KS, Mo IP, Rhee YO, Park KS, Oh KR. Studies on Haemophilus infection in chickens. I. Isolation of Haemophilus gallinarum from chicken affected with infectious coryza. Korean J Vet Res. 1981; 21:93–97.
18. Otsuki K, Iritani Y. Preparation and immunological response to a new mixed vaccine composed of inactivated Newcastle disease virus, inactivated infectious bronchitis virus, and inactivated Hemophilus gallinarum. Avian Dis. 1974; 18:297–304. PMID: 4853743.
19. Page LA. Haemophilus infections in chickens. I. Characteristics of 12 Haemophilus isolates recovered from diseased chickens. Am J Vet Res. 1962; 23:85–95. PMID: 14483162.
20. Quinn PJ, Markey BK, Carter ME, Donelly WJ, Leonard FC. Haemophilus species. Veterinary Microbiology and Microbial Disease. Oxford: Blackwell Science;2001. p. 147–151.
21. Sakamoto R, Kino Y, Sakaguchi M. Development of a multiplex PCR and PCR-RFLP method for serotyping of Avibacterium paragallinarum. J Vet Med Sci. 2012; 74:271–273. PMID: 21979456.
22. Sandoval VE, Terzolo HR, Blackall PJ. Complicated infectious coryza outbreaks in Argentina. Avian Dis. 1994; 38:672–678. PMID: 7832727.

23. Siddique AB, Rahman S, Hussain I, Muhammad G. Frequency distribution of opportunistic avian pathogens in respiratory distress cases of poultry. Pak Vet J. 2012; 32:386–389.
24. Soriano VE, Longinos GM, Fernández RP, Velásquez QE, Ciprián CA, Salazar-García F, Blackall PJ. Virulence of the nine serovar reference strains of Haemophilus paragallinarum. Avian Dis. 2004; 48:886–889. PMID: 15666870.
25. Soriano VE, Téllez G, Hargis BM, Newberry L, Salgado-Miranda C, Vázquez JC. Typing of Haemophilus paragallinarum strains by using enterobacterial repetitive intergenic consensus-based polymerase chain reaction. Avian Dis. 2004; 48:890–895. PMID: 15666871.
26. Yamaguchi T, Iritani Y, Hayashi Y. Hemagglutinating activity and immunological properties of Haemophilus paragallinarum field isolates in Japan. Avian Dis. 1989; 33:511–515. PMID: 2775098.
27. Yamaguchi T, Iritani Y, Hayashi Y. Serological response of chickens either vaccinated or artificially infected with Haemophilus paragallinarum. Avian Dis. 1988; 32:308–312. PMID: 3401174.
28. Zhao Q, Sun Y, Zhang X, Kong Y, Xie Z, Zhu Y, Zhou E, Jiang S. Evaluation of two experimental infection models for Avibacterium paragallinarum. Vet Microbiol. 2010; 141:68–72. PMID: 19729253.
Table 1
Summary of antibiotic sensitivity test against seven field isolates of Avibacterium paragallinarum using the disk diffusion test
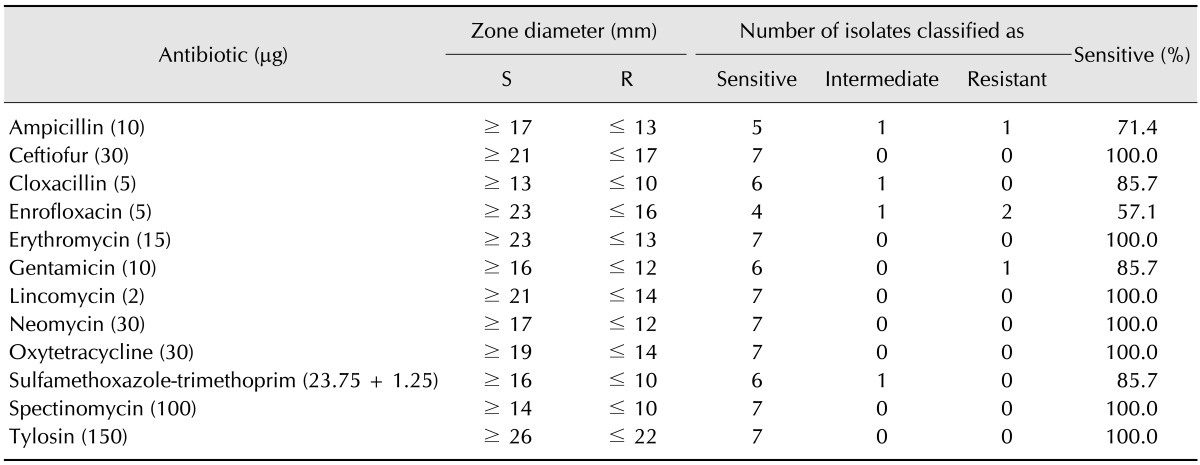
Table 2
Experimental design for the evaluation of efficacy of commercial infectious coryza (IC) vaccine (PoulShot Coryza) in the specific pathogen-free (SPF) chicken
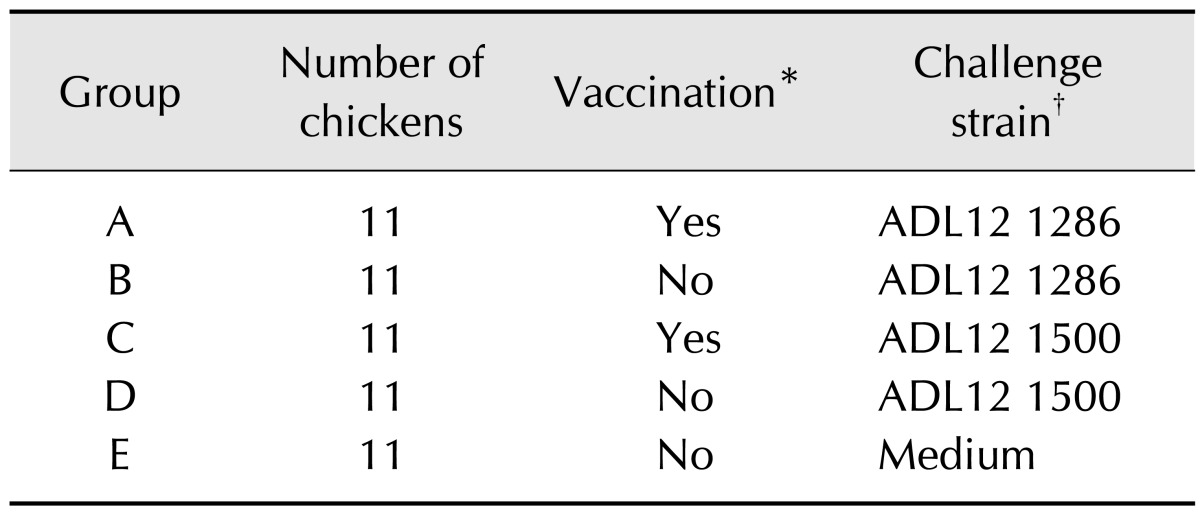
Table 4
Serological survey for serovar A of IC in chicken flocks with a history of distress or decreased egg production using the hemagglutination inhibition (HI) test
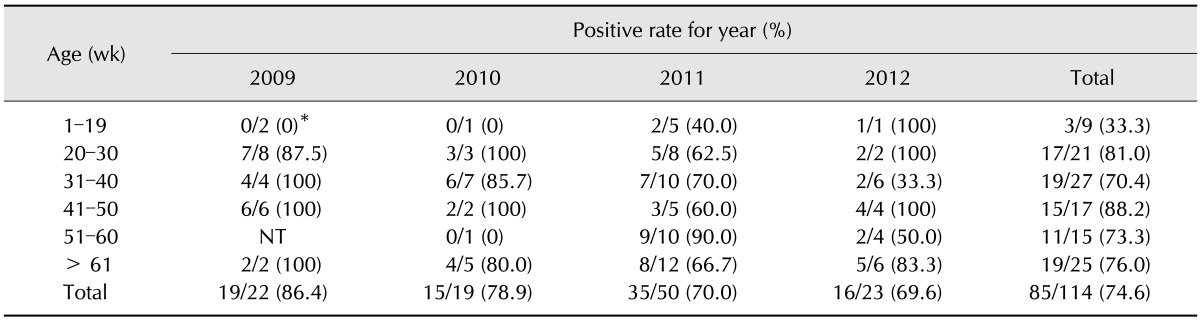
Table 5
Evaluation of pathogenicity used by clinical signs and re-isolation rate in SPF chickens challenged with a field strain of Avibacterium paragallinarum
*Group A and C were vaccinated at 40 days of age; Group A and B were challenged with field strain of ADL121286 at 54 days of age; Group C and D were challenged with field strain of ADL121500 at 54 days of age; Group E was the control group. †Average score of severity of clinical signs on a daily basis; Average score within row followed by dissimilar superscript letters are significantly different at p ≤ 0.05. ‡Number of positive samples/total number of samples tested (positive rate).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download