Abstract
Korean red ginseng (KRG) has long been used in traditional Korean and Oriental medicine. However, the anti-bacterial mechanism and therapeutic efficiency of KGR for intracellular Brucella infection are still unclear. In this study, the bactericidal activity of Korean red ginseng acidic polysaccharide (RGAP) on Brucella (B.) abortus and its cytotoxic effects on RAW 264.7 cells were evaluated. In addition, B. abortus internalization and intracellular replication in macrophages were investigated after RGAP treatment. RGAP-incubated cells displayed a marked reduction in the adherence, internalization and intracellular growth of B. abortus in macrophages. Furthermore, decreased F-actin fluorescence was observed relative to untreated B. abortus-infected cells. Western blot analysis of intracellular signaling proteins revealed reduced ERK, JNK and p38α phosphorylation levels in B. abortus-infected RGAP-treated cells compared to the control. Moreover, elevated co-localization of B. abortus-containing phagosomes with lysosome-associated membrane protein 1 (LAMP-1) were observed in RGAP-treated cells compared with the control. Overall, the results of this study suggest that RGAP can disrupt phagocytic activity of B. abortus via suppression of mitogen-activated protein kinases (MAPKs) signaling proteins ERK, JNK and p38 levels and inhibit intracellular replication of B. abortus by enhancing phagolysosome fusion, which may provide an alternative control of brucellosis.
Brucellosis is a major zoonotic problem worldwide and an economically important disease associated with severe illness in humans and abortion and sterility in many domestic and wild animals [61517]. The disease is caused by Brucella (B.) species, a small, non-motile, aerobic, Gram-negative coccobacillus that is highly contagious to humans and possesses the ability to invade and proliferate within host cells and evade the immune system, leading to chronic disease [1217]. The disease is also considered an occupational hazard to slaughterhouse workers, butchers and veterinarians because it can be transmitted via direct animal contact, inhalation, or the consumption of unpasteurized milk or other dairy products and undercooked meat products. The pathological manifestations include arthritis, endocarditis and meningitis [614].
The transmission of brucellosis varies with geographical conditions, climate, age, gender and species, but it is usually transmitted by sexually mature animals. After invading the lymphoid system, the pathogen proliferates within mononuclear phagocytes and disseminates in specific locations of the body, including the spleen, brain, heart and bones [2]. The development of an effective medical therapy for the disease is difficult because of its intracellular replication properties, which result in chronic infection and problems with multiple antibiotic regimens [11]. There are several obstacles to use of combinations of antibiotics for the treatment of this disease, including financial issues, therapeutic failures, and health and safety concerns. Therefore, the use of a safe alternative treatment, particularly natural plant products that can eliminate the various complications associated with brucellosis, must be explored.
Korean ginseng (the root of Panax ginseng Meyer) is a valuable medicinal herbs widely used as a supplementary herbal medicine for the treatment of cancer, diabetes and atherosclerosis [35]. The major active compounds of ginseng are ginsenosides, which have antidiabetic, anticancer and anti-inflammatory effects [3]. Other active constituents include acidic polysaccharides, which have been shown to possess either immunostimulatory or immunosuppressive effects depending on the length of treatment and disease environments [18]. Acidic polysaccharides are known to promote the production of cytotoxic cells against tumors, stimulate macrophages to produce T helper types 1 and 2 (Th1 and Th2), modulate antioxidant defense systems and suppress acute inflammatory responses at an early phase of Staphylococcus aureus infection [18]. However, the effects of acidic polysaccharides on the control of Brucella have not been explored.
In the present study, we investigated the effects of red ginseng acidic polysaccharide (RGAP) on invasion and intracellular survival inhibition in macrophages during B. abortus infection. The inhibitory effects suggest that this plant could be a promising alternative for the control and/or treatment of brucellosis.
Red ginseng acidic polysaccharide (RGAP) was isolated as previously described by the Institute of Technology, Korea Ginseng Corporation, Daejeon, Korea [10]. RGAP was stored as a dried powder at 4℃ until use. For the experiments, it was dissolved in sterile phosphate-buffered saline solution (PBS; pH 7.4) and filtered through 0.45 µm membranes (Minisart; Sartorius Stedim Biotech, Germany).
Murine macrophage RAW 264.7 cells (ATCC; TIB-71) were grown and prepared as previously described [11]. For all assays, macrophages were seeded on culture plates at a concentration of 1 × 105 cells per well and incubated overnight prior to infection.
The standard wild-type strains were derived from B. abortus 544 (ATCC 23448), a smooth, virulent B. abortus biovar 1 strain. The organism was cultivated in Brucella broth (Becton, Dickinson and Company, USA) or Brucella broth containing 1.5% agar (Becton, Dickinson and Company) at 37℃.
Bacteria (2 × 104 colony-forming units [CFU]/mL) were added to different concentrations of RGAP (0, 0.01, 0.1, 1, 2 and 4 mg/mL) and incubated at 37℃ for 0, 2, 4, 8 and 24 h. After incubation and dilution, 50 µL of each diluent was plated on Brucella agar and incubated at 37℃ for 3 days, after which bacterial survival rates were expressed as previously described [11].
RAW 264.7 cells were cultured in the presence of different concentrations of RGAP (0, 0.01, 0.1, 1, 1.5, 2, 4 mg/mL) in a 96-well cell culture plate for 48 h. Following incubation with RGAP, cytotoxicity was analyzed using a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) test as previously described [11].
RAW 264.7 cells were pretreated with the highest non-cytotoxic concentration of RGAP (0.1 mg/mL) or PBS (negative control) for 4 h prior to infection for analysis of the bacterial internalization efficiency. The bacteria were then deposited onto cells at multiplicities of infection (MOIs) of 100, centrifuged at 150 × g for 10 min at room temperature and incubated at 37℃ in 5% CO2 for 0 and 30 min. The infected cells were washed with PBS and then incubated at 37℃ in fresh medium containing fetal bovine serum (FBS) and gentamicin (30 µg/mL) for 30 min. Next, cells were washed using PBS and lysed with distilled water. Bacterial CFUs were measured by serial dilutions on Brucella agar plates. To measure the intracellular growth efficiency, bacterial infection was induced as described for bacterial internalization, after which infected cells were incubated at 37℃ for 1 h, washed with PBS, incubated on RPMI 1640 containing 10% (v/v) FBS and gentamicin (30 µg/mL) with RGAP (0.1 mg/mL) or PBS and then incubated for 2, 24 or 48 h. Finally, cells were washed and lysed as for the analysis of bacterial internalization efficiency.
RAW 264.7 cells were cultured in 12-well plates with 18 mm diameter glass coverslips (Fisher Scientific, Pittsburgh, PA). The cells were pre-incubated with RGAP (0.1 mg/mL) for 4 h, with cytochalasin D (0.5 mg/mL) added during the last 40 min of pre-incubation to inhibit bacterial internalization. The samples were then infected with B. abortus and centrifuged at 150 × g for 10 min at RT, after which they were incubated at 37℃ in 5% CO2 for 30 min. The cells were subsequently washed three times with PBS, fixed with 4% paraformaldehyde and incubated at 37℃ for 30 min. The adherent bacteria on the cell surface within 30 min of infection were monitored, after which the cells were washed three times with PBS and then permeabilized at −20℃ in methanol for 10 sec. The adherent bacteria were then stained with anti-B. abortus polyclonal rabbit serum (1 : 500) and fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin G (IgG) (1 : 500). The staining procedures were performed for 1 h at 37℃. Fluorescence images were collected using a microscope (Olympus IX70; Olympus, Japan) equipped with a digital camera (Nikon D5100; Nikon, Thailand) and analyzed as previously described [11].
RAW 264.7 macrophages were cultured and pretreated with RGAP as described for the bacterial adherence assay. The cells were then infected with FITC (Sigma-Aldrich, USA)-conjugated or unconjugated B. abortus. To observe F-actin reorganization, bacterial infection was monitored for 10 min as previously described [11]. Briefly, to detect co-localization of B. abortus-containing phagosomes (BCPs) with LAMP-1, bacterial infection with unconjugated B. abortus was performed for 1 h, after which cells were washed with medium and then incubated with fresh medium containing FBS, gentamicin and RGAP for 2 h. The infected cells were subsequently fixed, permeabilized with 0.1% Triton X-100 for 10 min at 22℃, and incubated with blocking buffer (2% goat serum in PBS) for 30 min. For F-actin staining, the cells were incubated with 0.1 µM rhodamine-phalloidin (Cytoskeleton, USA) for 30 min at 22℃. For LAMP-1 staining, all staining procedures were performed for 1 h at 37℃ using anti-LAMP-1 rat monoclonal antibody (1 : 100), Texas red-goat anti-rat IgG (1 : 1,000), anti-B. abortus rabbit serum (1 : 500) and FITC-conjugated goat serum (1 : 500). Blocking was performed using 2% goat serum in PBS for 30 min at 37℃. After final washing, the samples were mounted with DakoCytomation fluorescent mounting medium (Dako, USA) on microscope slides (Paul Marienfeld, Germany) and fluorescence images were collected using a laser scanning confocal microscope (FV1000; Olympus). Finally, data were processed using the FV10-ASW Viewer 3.1 software and analyzed as previously described [11].
RAW 264.7 cells were cultured in 6-well plates and pretreated with RGAP as described in the bacterial adherence assay. The cells were infected for 30 min, fixed, permeabilized and stained as previously described [11]. The F-actin content was quantified using a FACSCalibur flow cytometer (BD Biosciences, USA). All experiments were performed in duplicate and repeated at least three times.
Immunoblot analysis was conducted as described by MacPhee [13]. Briefly, RAW 264.7 cells were cultured in 6-well plates, treated with 0.1 mg/mL RGAP and infected with B. abortus for the indicated times. The cells were then washed twice with ice-cold PBS and lysed using ice-cold radioimmunoprecipitation assay (RIPA) buffer with 1% protease inhibitor cocktail for 30 min at 4℃. The protein concentration was then measured using a Bradford protein assay (Bio-Rad Laboratories, USA). Next, samples were separated by SDS-PAGE and transferred onto an Immobilon-P membrane (Millipore, USA) using 1× transfer buffer (25 mM Tris, 192 mM glycine and 20% methanol) as previously described [11]. The immunoblot signals were quantified using the ImageJ software (National Institutes of Health, USA).
The data are expressed as the means ± standard deviation (SD) for replicate experiments. Statistical analysis was conducted using the GraphPad Prism software (ver 4.00; GraphPad Software, USA). A Student's t-test or one-way ANOVA was used for statistical comparisons between groups. A p < 0.05 was considered statistically significant.
The bacterial survival rates did not differ upon incubation with various concentrations of RGAP (0.01, 0.1, 1, 2 and 4 mg/mL), indicating that RGAP had no bactericidal effect against B. abortus at any of the concentrations used in the experiment. When RAW 264.7 cells were incubated for 48 h with various concentrations of RGAP (0, 0.01, 0.1, 1, 1.5, 2, 4 mg/mL), the OD values of the cells cultured at concentrations from 1 to 4 mg/mL RGAP decreased markedly. However, the OD values observed in the presence of RGAP at 0.1 mg/mL did not decrease significantly compared with the OD values of untreated control cells. These results indicate that RGAP did not have any cytotoxic effects on RAW 264.7 cells at ≤ 0.1 mg/mL. Therefore, the highest non-cytotoxic concentration of RGAP (0.1 mg/mL) was used for the subsequent experiments.
The invasion of B. abortus in RAW 264.7 cells was verified by pre-incubation with the highest non-cytotoxic concentration of RGAP (0.1 mg/mL) for the indicated times (0, 30 min). The results indicate that the invasion of B. abortus into RGAP-treated cells was significantly reduced compared with the untreated control (p < 0.05) (panel A in Fig. 1). Furthermore, RGAP-treated cells displayed significantly reduced intracellular growth of B. abortus at 2, 24 and 48 h post-infection compared with the untreated control cells (panel B in Fig. 1).
The adherence of B. abortus on RAW 264.7 cells was significantly reduced by pre-incubation with RGAP (28 ± 2) relative to untreated control cells (53 ± 2.65), showing a reduction rate of 47.17 ± 1.08% (p < 0.001) (Fig. 2).
Phalloidin-associated F-actin fluorescence microscopy indicated diminished F-actin polymerization for B. abortus invasion in RGAP-treated cells compared with untreated control cells and showed a reduction in filopodia and lamellipodia scattering in the peripheral cells (panel A in Fig. 3). Because F-actin polymerization is required for B. abortus phagocytosis, the F-actin content of RGAP-treated cells upon B. abortus invasion was assessed and quantified. Fluorescence-activated cell sorting (FACS) analysis revealed that treatment of murine macrophages with RGAP significantly reduced the F-actin content in these cells upon bacterial infection, as indicated by decreased F-actin fluorescence intensity compared with B. abortus-infected untreated cells (panel B and C in Fig. 3). The F-actin content of the uninfected RGAP-treated cells showed no significant difference from the untreated control cells.
The phosphorylation levels of ERK1/2, JNK and p38α in RGAP-treated cells at 30 min post-infection were reduced by 27.58%, 34.76% and 47.92%, respectively, compared with B. abortus-infected control cells (Fig. 4). These findings indicate that RGAP suppressed the activation of MAPKs in RAW 264.7 cells, which inhibited the invasion of B. abortus into macrophages. In addition, co-localization of LAMP-1 with BCPs in RGAP-treated cells was observed to markedly increase, showing a significant increase of up to 2.03-fold after 2 h of incubation relative to untreated control cells (p < 0.001) (Fig. 5).
Brucella spp. are highly infectious to humans, with an annual incidence of approximately 500,000 cases worldwide [617]. Furthermore, there is no safe, licensed and effective vaccine currently available to control human brucellosis. Consequently, the application of traditional medicine and natural products to control brucellosis should be explored.
Korean ginseng (the root of Panax ginseng Meyer), which is one of the most valuable medicinal herbs, is mainly used to maintain homeostasis in the body. Some of its identified pharmacological efficacy involves improved brain function, pain-relieving effects, antitumor activity, anti-stress effects and anti-aging effects [5]. One of its reported active ingredients is acidic polysaccharide, which has been shown to have immunostimulatory functions including up-regulation of the functional roles of natural killer cells and macrophages linked to antitumor activities in several studies [3]. Although ginseng has been shown to improve microbial clearance from the body and studies have shown that acidic polysaccharide can be used as a remedy for influenza viral infection, its potential therapeutic efficacy against Brucella has not been clinically examined [818].
Microbial adhesion to host cells is essential to successful establishment of infection, and is crucially dependent on specific cell-to-bacterial interactions mediated by specific cell surface adhesion molecules. Studies have shown that ginseng polysaccharides can interrupt microbial adhesion to host cells, thereby preventing the establishment of infectious diseases including Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans, Propionibacterium acnes and Staphylococcus aureus [8]. In the present study, we examined the effect of RGAP on B. abortus adhesion to phagocytic cells. In agreement with previous studies, our results showed that RGAP interferes with the adherence of B. abortus to the macrophage cell membrane in vitro, suggesting that it could inhibit the adherence of B. abortus to macrophage surface membranes during infection. Consistent with the interfering effect of RGAP on the adherence of B. abortus to macrophages, the invasion of this pathogen in RGAP-treated cells also decreased significantly when compared to the untreated control. These findings indicate that RGAP has an inhibitory effect on B. abortus phagocytosis by inhibiting F-actin polymerization, which is required for the uptake of these microorganisms into the host cell.
A significant reduction in the number of Staphylococcus aureus recovered in the spleen, kidneys and blood of mice and protection from lethal sepsis upon treatment with acidic polysaccharide was observed [1]. The bacterial load and lung pathology caused by Pseudomonas aeruginosa in rats that were treated with an aqueous extract of ginseng were also reduced [16]. Therefore, RGAP could account for the inhibitory effects of Korean red ginseng against Brucella infection. Mitogen-activated protein kinase (MAPK) has also been shown to play an important role in bacterial phagocytosis and actin cytoskeleton remodeling [9]. The treatment of peritoneal macrophages with acidic polysaccharides after subsequent exposure to heat-killed S. aureus reduced the expression of phospho-JNK1/2 and p38 MAPK [1]. In the present study, we investigated the phosphorylation of MAPKs in macrophages in the presence or absence of RGAP during B. abortus invasion. In agreement with the previous study, our findings revealed that RGAP reduced the expression of MAPKs (ERK1/2, JNK and p38α) in B. abortus-infected macrophages. Consequently, these findings indicate that the inhibitory effects of RGAP on MAPKs-linked phagocytic signaling pathways could disrupt F-actin polymerization and subsequent B. abortus invasion in macrophages.
In our study, the incubation of macrophages with RGAP after B. abortus infection significantly reduced intracellular survival of B. abortus. One of the strategies for the intracellular survival of virulent B. abortus is its ability to progressively inhibit phagosome-lysosome fusion followed by maturation of Brucella-containing phagosomes (BCPs) into replicative phagosomes or brucellosomes [4]. Therefore, we investigated the involvement of RGAP in B. abortus intracellular trafficking in macrophages by demonstrating its effect on the interaction of BCPs with LAMP-1. LAMPs are transmembrane proteins with a large, heavily glycosylated luminal domain and a short cytosolic tail required for fusion of lysosomes with phagosomes, which form organelles with degradative and antimicrobial components essential for the killing of internalized microorganisms [7]. In this study, LAMP-1 staining revealed enhanced phagosome-lysosome fusion of B. abortus for up to 48 h, which suggests that RGAP inhibits bacterial intracellular replication by enhancing early and late phagosome-lysosome fusion of Brucella within macrophages.
In conclusion, our results suggest that RGAP modulates B. abortus pathogenesis by inhibiting phagocytosis and intracellular replication in macrophages. These findings will facilitate development of a safe and economical alternative approach to the prevention and/or control of brucellosis in livestock industries and humans that can be applied as a component in feed additives or in natural food products and medicines.
Figures and Tables
Fig. 1
Effect of red ginseng acidic polysaccharide (RGAP) on invasion and intracellular growth of Brucella (B.) abortus. (A) Bacterial internalization efficiency. (B) Bacterial intracellular growth efficiency. Data represent the means ± SD of duplicate samples in at least three independent experiments. Statistically significant differences relative to the untreated control are indicated by asterisks (*p < 0.05; **p < 0.01; ***p < 0.001).

Fig. 2
Effect of RGAP on the adherence of B. abortus to macrophages. Fluorescence images were collected using a microscope equipped with a camera. One hundred macrophages were selected at random, and the bacteria that adhered to the cells were counted. Data represent the mean ± SD of triplicate samples in at least three independent experiments. Statistically significant differences relative to the untreated control are indicated by asterisks (***p < 0.001).
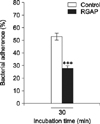
Fig. 3
Fluorescence-activated cell sorting (FACS) analysis of the effects of RGAP on phagocytosis of B. abortus by F-actin polymerization modulation. (A) F-actin polymerization and bacterial co-localization (scale bars = 5 µm). (B) FACS analysis for F-actin content. (C) Intensification of F-actin polymerization. The data shown are representative of at least three independent experiments. Statistically significant differences relative to untreated control cells are indicated by an asterisk (*p < 0.05). DIC, differential interference contrast.
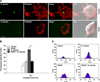
Fig. 4
Effect of RGAP on activation of intracellular signaling for the phagocytosis of B. abortus. Immunoblot analyses of total RAW 264.7 cell lysates pre-treated with RGAP were assessed using phospho-specific ERK1/2, JNK and p38α antibodies at the indicated times. Images shown are representative of at least three independent experiments.
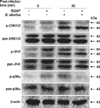
Fig. 5
Effect of RGAP on intracellular trafficking of B. abortus. One hundred bacteria within macrophages were randomly selected, and the extent of LAMP-1 acquisition by the bacteria was determined. Data represent the means ± SD of triplicate samples. Statistically significant differences relative to the untreated control are indicated by asterisks (***p < 0.001).
Acknowledgments
The present work was supported by a grant from the Ministry of Education, Basic Science Research Program, National Research Foundation, Korea (2015-0885).
References
1. Ahn JY, Song JY, Yun YS, Jeong G, Choi IS. Protection of Staphylococcus aureus-infected septic mice by suppression of early acute inflammation and enhanced antimicrobial activity by ginsan. FEMS Immunol Med Microbiol. 2006; 46:187–197.

2. Al-Mariri A, Saour G, Hamoud R. In vitro antibacterial effects of five volatile oil extracts against intramacrophage Brucella abortus 544. Iran J Med Sci. 2012; 37:119–125.
3. Byeon SE, Lee J, Kim JH, Yang WS, Kwak YS, Kim SY, Choung ES, Rhee MH, Cho JY. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean red ginseng. Mediators Inflamm. 2012; 2012:732860.

4. Carvalho Neta AV, Mol JPS, Xavier MN, Paixão TA, Lage AP, Santos RL. Pathogenesis of bovine brucellosis. Vet J. 2010; 184:146–155.

5. Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008; 29:1109–1118.

6. Gul ST, Khan A. Epidemiology and epizootology of brucellosis: a review. Pak Vet J. 2007; 27:145–151.
7. Huynh KK, Eskelinen E, Scott CC, Malevanets A, Saftig P, Grinstein S. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J. 2007; 26:313–324.

8. Kang S, Min H. Ginseng, the 'immunity boost': the effects of Panax ginseng on immune system. J Ginseng Res. 2012; 36:354–368.

9. Kim DG, Simborio HLT, Reyes AWB, Min W, Lee HJ, Lee JJ, Kim S. The key roles of toll-like receptor (TLR) for intracellular survival of Brucella. J Prev Vet Med. 2013; 37:185–192.

10. Kwak YS, Kyung JS, Kim JS, Cho JY, Rhee MH. Anti-hyperlipidemic effects of red ginseng acidic polysaccharide from Korean red ginseng. Biol Pharm Bull. 2010; 33:468–472.

11. Lee JJ, Kim DH, Kim DG, Lee HJ, Min W, Rhee MH, Yun BS, Kim S. Phellinus baumii extract influences pathogenesis of Brucella abortus in phagocyte by disrupting the phagocytic and intracellular trafficking pathway. J Appl Microbiol. 2013; 114:329–338.

12. Lee JJ, Kim DH, Park SB, Lim JJ, Kim DG, Min WG, Lee HJ, Kim DK, Chang HH, Kim S. Redundant effects of ketamine on the pathogenesis and severity of Brucella abortus infection. Comp Immunol Microbiol Infect Dis. 2013; 36:71–81.

13. MacPhee DJ. Methodological considerations for improving Western blot analysis. J Pharmacol Toxicol Methods. 2010; 61:171–177.

14. Oliveira SC, de Almeida LA, Carvalho NB, Oliveira FS, Lacerda TLS. Update on the role of innate immune receptors during Brucella abortus infection. Vet Immunol Immunopathol. 2012; 148:129–135.

15. Robertson GT, Roop RM Jr. The Brucella abortus host factor I (HF-I) protein contributes to stress resistance during stationary phase and is a major determinant of virulence in mice. Mol Microbiol. 1999; 34:690–700.

16. Song Z, Johansen HK, Faber V, Moser C, Kharazmi A, Rygaard J, Høiby N. Ginseng treatment reduces bacterial load and lung pathology in chronic Pseudomonas aeruginosa pneumonia in rats. Antimicrob Agents Chemother. 1997; 41:961–964.

17. Ullah RW, Shirazi JH, Abubakar M, Zahur AB, Latif A, Alam T. Genetic diversity, zoonotic risk and "One Health" initiative of bovine brucellosis. Res J Vet Pract. 2013; 1:5–9.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download