Abstract
Severe fever with thrombocytopenia syndrome (SFTS) caused by the SFTS virus (SFTSV), a phlebovirus in the family Bunyaviridae, is an emerging tick-borne infectious disease that impacts humans. This disease manifests as a decreased blood cell count and multi-organ failure, with a case-fatality rate of more than 12% in China. Because vaccines or antiviral drugs for the treatment of this disease are not available, monitoring the SFTS circulation in animals and controlling the tick-mammal cycle are important for preventing SFTS. Monoclonal antibodies against the recombinant nucleoprotein of SFTSV were generated to develop a competitive enzyme-linked immunosorbent assay (cELISA) for the detection of antibodies against SFTSV infection in cattle. The specificity and sensitivity of cELISA was assessed by comparing the results of this assay to those of an immunofluorescence assay (IFA). The results of the cELISA using 416 field bovine serum samples and laboratory-immunized positive sera showed 98.1% consistency with those of the IFA. The cELISA used in this study did not show cross-reactivity with antisera against other viral cattle diseases. The cELISA presented in this study can be applied to detect antibodies against SFTSV in cattle.
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging tick-mediated infectious disease that was recently discovered in the rural areas of China [24]. The clinical characteristics of this disease in humans primarily include thrombocytopenia, leukocytopenia, high fever, gastrointestinal symptoms, and imbalanced liver enzyme production [21]. The pathogen responsible for SFTS is the SFTS virus (SFTSV), a novel Phlebovirus in the family Bunyaviridae [21]. This virus contains three RNA genome segments coated with nucleoprotein (NP) that are encapsulated in an 80- to 120 nm diameter glycoprotein viral envelope [2124]. Since the first report of human SFTS in central China, additional cases have been reported continuously in other parts of China, Korea, and Japan [172526]. In 2013, 36 patients were diagnosed with SFTS, and the fatality rate of this disease was 47.2% in Korea [612].
Among viral pathogen species known to cause human diseases, 76.0% also infect other vertebrate animals [1819]. Moreover, a 41.8% seropositive rate against SFTSV has been reported in domestic animals in China, strongly suggesting that animals are major reservoirs of tick-mediated SFTSV transmission [134]. In addition, seropositive shrews and rodents were recently implicated as potential animal hosts susceptible to SFTSV [9]. A study in Korea showed that SFTSV-positive ticks are widespread throughout most provinces, particularly during summer [13]. Moreover, recent evidence has shown that SFTSV acts as a zoonotic pathogen, and further studies of serological prevalence in farming or wild animal species are necessary.
Reverse transcriptase (RT)-polymerase chain reaction (PCR)-based methods have been conducted to detect the SFTSV antigen in blood samples [716]. Moreover, immunofluorescence assays (IFA) are frequently used to detect SFTSV-specific antibodies in the sera of SFTS patients [23]. These diagnostic methods are associated with several disadvantages, such as the need to handle the live virus, which requires special facilities with high-level biosafety equipment. Furthermore, these methods are laborious when applied to large numbers of samples [21]. In-house indirect enzyme-linked immunosorbent assays (ELISA) and double-antigen sandwich ELISA have been developed to detect immunoglobulins against the NP of SFTSV [5].
Here, we report the development of a competitive ELISA (cELISA) based on the competition of SFTSV-specific antibodies in the test serum with monoclonal antibodies (mAbs) against the NP of SFTSV. The NP recombinant protein is suitable for use as a diagnostic antigen as it is a highly immunogenic protein that has been shown to be expressed from the early stage of virus infection in other Bunyaviridae cases [101123]. We applied this technique to field bovine sera and experimentally generated bovine antisera against SFTSV and investigated the correlation and consistency of this assay with IFA. The cELISA presented in this study can be used to detect antibodies against SFTSV in cattle.
For antigen production, the gene encoding the full-length NP protein of SFTSV strain LN3 (GenBank accession No. HQ141612) was expressed in a bacterial overexpression system. The S fragment of SFTSV was chemically synthesized using the Bioneer gene synthesis service (Bioneer, Korea). The 735 bp gene encoding N-protein was amplified using the following primers: SFTS-NP-1 CTCGGAATTCACATGTCA GAGTGGTCC and SFTS-NP-735 CTTCAAGCTTCAGGTT CCTGTAAGCAG. The PCR amplification was performed using a T3000 thermocycler (Biometra, Germany). The NP gene was cloned into pET-30a(+) (Invitrogen, USA) using the BamHI and XhoI restriction enzymes (New England Biolabs, UK), and subsequently transformed into Escherichia coli BL21 (DE3) (Yeastern Biotech, Taiwan) to express 6xHis-tagged fusion proteins. Following induction with 0.2 mM isopropyl β-D-1-thiogalactopyranoside (AMRESCO, USA) for 20 h at 25℃, the bacterial cell pellet was sonicated in chromatography buffer (20 mM sodium phosphate, 500 mM NaCl, 8 M Urea, and 20 mM imidazole, pH 7.4) and purified using Ni-NTA agarose (Qiagen, Germany) [22]. The affinity-purified protein was urea gradient dialyzed prior to use. The recombinant NP protein was solubilized in 8 M urea buffer, which was then exchanged for 150 mM Tris-HCl (iNtRon Biotechnology, Korea) for further use in subsequent experiments.
Animal experiments were conducted according to the protocol of the Institutional Animal Care and Use Committee of the Republic of Korea. Rabbit polyclonal antibody and mouse mAbs were generated after four immunizations with recombinant NP. Polyclonal antibodies were produced in rabbits after four injections of 500 µg of the NP antigen mixed with adjuvant at two-week intervals. The NP-specific mAbs were synthesized as previously described [2]. Briefly, eight mice were injected four times with 100 µg of recombinant NP. Following immunization, the mouse spleen cells were collected and fused with myeloma cells to generate mAbs. Among the mouse hybridoma cell lines, 14 clones were selected according to specific interactions with NP using indirect ELISA.
The cattle were immunized with an injection of formalin-inactivated SFTSV. Briefly, 60 mL of 108 TCID50/mL of the SFTSV was inactivated using 0.025% formalin solution for 72 h. The inactivated virus was concentrated fivefold via filtration using a 10K Ultracel (Millipore, Germany). The loss of viral infectivity was confirmed by culture in Vero cells. Two cattle aged 4 months were used to produce positive control sera against SFTSV. Approximately 2 mL of inactivated SFTSV was emulsified in gel-based adjuvant, and the cattle received intramuscular injections every 2 weeks, with a total of three 3 injections. Serum samples were collected 2 weeks after the last antigen injection. The serum used as a negative control in IFA and cELISA was collected from cattle prior to immunization.
IFA was performed as previously described, with slight modification [23]. Briefly, Vero E6 cells grown to 80% confluency in 150 cm2 were infected with 2 mL of 1 × 103 TCID50/mL SFTSV. After 6 days, the infected cells (~3,000 cells per well) were spotted onto the microscope slides with reaction wells (Paul Marienfeld, Germany). The cells were immediately fixed with a methanol : acetone (1 : 1) solution for 30 min, after which non-specific signals were blocked with 5% horse serum in a total volume of 20 µL per well. The cells were incubated with mouse mAbs, α-NP specific polyclonal rabbit sera or field bovine serum samples for 1 h at room temperature, followed by detection using fluorescein isothiocyanate (FITC)-labeled secondary antibodies. The FITC-conjugated anti-mouse, rabbit, and bovine antibodies (KPL, USA) were diluted to a concentration of 2.5 µg/mL prior to use. The cells were observed using a Nikon TE-2000U fluorescence microscope (Nikon, Japan).
cELISA was performed as previously described, with slight modification [8]. Briefly, a Polysorp ELISA plate (Nunc, USA) was coated with 50, 100 or 200 ng/well of recombinant NP. The plate was then incubated for 16 hours at 4℃ to allow antigen binding. After washing with 0.5% Tween 20 (Sigma-Aldrich, USA) in 1× PBS to remove the unbound antigens, the plate was blocked with 5% skim milk and 1% horse serum in 1× PBS. For the serological test, 50 µL of diluted test sera and the same amount of HRP-conjugated mAb (1 : 500) were added to the antigen coated plate and incubated for 90 min at 37℃. The mAb was conjugated with HRP according to the instructions provided with the EZ-LinkTM Plus Activated Peroxidase kit (Thermo Fisher Scientific, USA). After washing the plate six times, 3,3',5,5'-tetramethylbenzidine peroxidase substrate (KPL) was added to develop the color. The reaction was terminated with 1 M sulfuric acid (Sigma-Aldrich). The optical density (OD) of the sample was calculated as follows: the OD at 450 nm was subtracted from the OD at 630 nm to adjust for the background absorbance. The OD values were converted to percent inhibition (PI) values using the following formula: PI =[1 – (OD test sample/OD negative control)] × 100.
Bovine field serum samples (n = 416) were collected from breeding cattle in the southern part of Korea (Gyeongsang province) from September 2013 to August 2014.
cELISA was conducted using antisera against different viruses: bovine viral diarrhea (BVD) type 1 virus neutralization positive control serum, BVD type 2 virus neutralization positive control serum (National Veterinary Services Laboratories, USA), bovine coronavirus, bovine rotavirus, Bovine leukemia virus and Akabane virus (93FMX) (generated in the Animal and Plant Quarantine Agency, Korea).
The positive and negative groups were compared by one-way ANOVA and the Mann-Whitney rank sum test, with p values ≤ 0.001 indicating a significant difference between groups (SYSTAT ver. 4.0; Systat Software, USA). Bee swarm and box plots indicating the distributions of negative or positive groups were generated using an online application [14]. Other graphs and receiver operating characteristic (ROC) [27] curves were generated using the Excel graph function.
The gene for the recombinant NP was amplified from the commercially synthesized S fragment of SFTSV (GenBank accession No. HQ141612) and cloned into a protein expression vector. A 34 kDa recombinant protein band was well maintained after all purification procedures (Fig. 1). This recombinant NP was used as the antigen to generate polyclonal and mAbs. NP-specific polyclonal antibody was obtained in rabbits after four antigen immunizations, and the titer of the antibody was tested by IFA and Western blotting (Fig. 2). NP-specific mAbs were produced from the spleen hybridoma cells of immunized mice, and the three clones (6D55, 8F31, and 10G7) showed strong interactions in the IFA and Western blot tests; therefore, these clones were used as competitive antibodies to develop the cELISA. A clear band corresponding to the size of NP, which appeared only after SFTSV infection, was observed upon Western blot analysis with all generated antibodies (panel A in Fig. 2). Moreover, IFA using these antibodies showed intense cytoplasmic staining, specifically in SFTSV-infected Vero cells (panel B in Fig. 2).
SFTSV-positive cattle sera were generated from two cattle that were injected three times with inactivated SFTSV. Serially diluted positive or negative serum samples were tested to determine the optimized dilution rate to distinguish SFTSV-positive serum samples from negative samples in IFA. The maximum dilution rate of serum for the IFA at which a non-specific background signal was not observed in the negative control was 1/80 (Fig. 3).
ELISA plates coated with different concentrations of antigen were used to determine the optimal coating antigen concentration (Supplementary Fig. 1). Among the mAb clones screened based on IgG responses in the IFA and Western blot tests, 10G7 was selected as the competitive antibody for cELISA because this mAb showed stable reactivity after the conjugation of horseradish peroxidase (HRP) protein. The 1 : 500 diluted mAb-peroxidase conjugate yielded the maximum percent inhibition rate in the cELISA (Supplementary Fig. 1).
The immunized cattle serum samples were tested for the cELISA. Both 1 : 2 and 1 : 5 dilutions of the positive serum samples showed higher PI values than the negative serum. However, a 1 : 5 dilution of the positive control sera yielded a large difference in the optical density (OD) between the positive and negative serum samples (Fig. 4). The repeatability of this finding was confirmed by three independent experiments.
The specificity of the SFTSV cELISA was examined using antisera against different types of viruses from cattle. The maximum PI value of laboratory-immunized bovine serum in the cELISA was 93%, while other antisera against different viruses, such as BVD type 1 and BVD type 2, bovine coronavirus, bovine rotavirus, Bovine leukemia virus and Akabane virus (93FMX), yielded PI values of – 18 to 11% (Supplementary Fig. 2). These findings demonstrate that the cELISA is highly specific for the detection of antibodies against SFTSV.
A total of 416 bovine serum samples and two positive control sera were tested with the IFA to distinguish negative serum samples from SFTSV. The results showed that 97.4% (407/418) of the bovine serum samples were negative, while 2.6% (11/418) of the bovine serum samples were positive according to the IFA. cELISA was performed using the SFTS-negative group samples, and the mean inhibition percent was 11.3% with a standard deviation of 12.7%. Therefore, we determined the cut-off value to be 49.5% (mean ± 3SD) (Fig. 5). The cELISA and IFA were compared using this cut-off value, and 98.1% (410/418) consistency was observed between results (Table 1). The accuracy of cELISA was demonstrated with a plot of the distribution of PI values obtained from the IFA-negative or positive cattle group (Fig. 6).
Because official standard methods for the antibody detection of SFTSV have not been determined, the cut-off criteria determination for IFA requires further optimization. Therefore, ROC curve was employed to determine the sensitivity and specificity and define the optimal cut-off value for IFA. The ROC curves of four different IFA cut-off values were compared, and the results showed that a 1/80 dilution of the sample strongly supports the result of cELISA. For this dilution, the area under the curve (AUC) was 0.91. While a 1/128 dilution rate as an IFA cut-off point maximizes the specificity and sensitivity compared with other dilution rates, this condition is too strict to detect positive samples above the strict threshold in cELISA. The other IFA cut-off conditions (1/16 and 1/64) were also excluded because they showed moderate AUC values (Fig. 7).
Using a 1/80 dilution as cut-off for the IFA and a PI of 49.5% as a cut-off for cELISA, 0.95% (4/418) of the bovine serum samples were identified as positive, while 97.1% (406/418) of the serum samples were negative.
Tick-mediated SFTSV is an emerging infectious virus identified in 2009 that shows 12% lethality in humans [23]. However, the range of host species or virus circulation routes in the environment is difficult to determine because a standard diagnosis method is not available. Therefore, a simple and effective method of detecting SFTSV antibody is needed to identify potential hosts and develop anti-viral strategies. In an effort to detect SFTSV-specific antibodies, in-house ELISA and double-antigen sandwich ELISA (DAS-ELISA) assays were developed [524]. Specifically, DAS-ELISA was used to determine the seroprevalence of humans and animals in China. A multiplexed Luminex-based immunoassay method was also established for high-throughput detection [20]. These newly developed techniques are highly specific and sensitive, but are not commercially available, and some of these methods require special equipment for diagnosis.
Here, we report the development of cELISA using mAb to detect antibodies against SFTSV. The cELISA format was selected as a serodiagnostic test that has value in handling field serum samples because it requires only small volumes of serum for diagnosis and shows species flexibility. Immunostaining and Western blotting were used to detect mAbs with high affinity to SFTSV-infected Vero cells. The results indicated that the generated mAbs were appropriate for use in the development of a cELISA.
A previous study showed that the involvement of domestic animal husbandry is a significant factor in SFTSV infection when compared with outdoor activities or tick exposure in China [15]. Therefore, we collected 416 field bovine sera from farms in Gyeongsang Province in Korea, where 30.6% (11/36) of human SFTSV infections were reported in 2013 [12]. First, we tested the field bovine serum samples using IFA. The bovine serum samples that yielded negative results in the IFA were used as a negative group to determine the cut-off value for the cELISA. All field serum samples and two experimentally immunized bovine sera were examined for an immune response to SFTSV using both IFA and cELISA.
The results showed that 97.4% of the total serum samples yielded negative results in the IFA; therefore, this group was regarded as negative to SFTSV. The cELISA cutoff value was subsequently calculated using the mean inhibition rate of the IFA-negative population ± 3S.D. (cutoff PI = 49.5%). Using this cut-off, 99.8% of the IFA-negative bovine serum samples were also considered to be negative in the cELISA. Although the newly developed cELISA is highly specific, more field animals infected with SFTSV would need to be measured to improve the sensitivity.
SFTSV was first isolated in goats, after which antibodies against SFTSV were detected in the sera obtained from goats at one goat farm in the southern part of Korea (Gyeongsang province) in September 2014 (personal communication with Dr. Lee, Yoon-Hee and Mr. Choi, Jeong-Soo, Foreign Animal Disease Division, Animal and Plant Quarantine Agency, Korea). Serological diagnostic methods are necessary to test large numbers of animals. While the cELISA developed in the present study requires additional tests to validate its performance, the results observed to date are promising. Further studies to adapt this diagnostic technique to a broad spectrum of animal species are currently in progress.
Figures and Tables
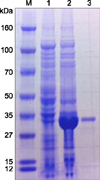 | Fig. 1Purified recombinant full-length nucleoprotein (NP) of severe fever with thrombocytopenia syndrome virus (SFTSV) stained with Coomassie brilliant blue after SDS-PAGE. A recombinant protein band of approximately 34 kDa was expressed after isopropyl β-D-1-thiogalactopyranoside (IPTG) induction. A single band remained after the affinity-specific purification. Lane 1, Escherichia (E.) coli BL21 (DE3) of the cell lysate before induction; Lane 2, E. coli BL21 (DE3) cell lysate after 20 h of induction with 0.2 mM IPTG; Lane 3, 100 ng of purified protein. |
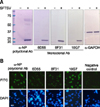 | Fig. 2The reactivity of rabbit polyclonal antibody and mouse monoclonal antibodies (mAbs) against the NP in Western blot and immunofluorescence assay (IFA). (A) Western blot analysis of one polyclonal antibody and three mAbs in Vero cells before and after SFTSV infection. NP expression was assessed at 6 days post infection (dpi = 6), and mock-infected cells were included. Each cell lysate was separated on an 8–16% gradient SDS-PAGE gel and transferred to membranes for Western blot analysis. The α-GAPDH antibody was used as a loading control. (B) Rabbit anti-NP and mouse anti-SFTSV polyclonal antibodies were diluted 1 : 1,000 for immunoblotting and 1:200 for IFA. Three mAbs were diluted 1 : 1,000 for immunoblotting and 1 : 50 for IFA, and the secondary antibodies for rabbit and mouse IgG were diluted 1 : 1,000 for immunoblotting and 1 : 200 for IFA. DAPI staining (4',6-diamidino-2-phenylindole) was used to stain the cell nuclei. |
 | Fig. 3Antibody responses from laboratory immunized cattle. The SFTSV-positive bovine serum was tested in the IFA. The optimized dilution rate of the positive serum samples was 1/80, while less diluted sera (1/10 and 1/50) showed strong background signals in the IFA. |
 | Fig. 4The percent inhibition (PI) value and optical density (OD) of laboratory immunized bovine sera in a competitive enzyme-linked immunosorbent assay (cELISA). Serum samples collected from two immunized cattle and one negative control cattle were diluted from 1/2 to 1/300 and tested for (A) PI value and (B) OD value (at 450–630 nm) in cELISA. Error bars represent the standard error of the mean of independent experiments repeated at least three times. |
 | Fig. 5Establishment of cut-off PI value in cELISA. Frequency distributions of the PI values for 407 bovine serum samples determined as SFTSV-negative in IFA (1 : 80 dilution). The cut-off of 49.5% (mean ± 3SD) is indicated with the arrow. |
 | Fig. 6Scatter dot plot indicating individual and mean values of PI value in cELISA. The scatter dot plot represents the distribution of antibody titers to the NP of SFTSV in IFA-negative (n = 407) and IFA-positive (n = 11) samples. The center lines show the medians; box limits indicate the 25th and 75th percentiles as determined by R software; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles, with outliers represented as dots; and the data points are plotted as open circles. n = 407 and 11 sample points. |
 | Fig. 7Receiver operating characteristic (ROC) for analysis of the cELISA. ROC curves for cELISA compared with four different IFA cut-off points. AUC, area under the curve. |
Acknowledgments
This study was funded by the Ministry of Agriculture, Food and Rural Affairs, Republic of Korea to the Animal and Plant Quarantine Agency.
References
1. Baneth G. Tick-borne infections of animals and humans: a common ground. Int J Parasitol. 2014; 44:591–596.

2. de StGroth SF, Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980; 35:1–21.

3. Ding S, Yin H, Xu X, Liu G, Jiang S, Wang W, Han X, Liu J, Niu G, Zhang X, Yu XJ, Wang X. A cross-sectional survey of severe fever with thrombocytopenia syndrome virus infection of domestic animals in Laizhou city, Shandong province, China. Jpn J Infect Dis. 2014; 67:1–4.

4. Jiang XL, Wang XJ, Li JD, Ding SJ, Zhang QF, Qu J, Zhang S, Li C, Wu W, Jiang M, Liang MF, Bi ZQ, Li DX. Isolation, identification and characterization of SFTS bunyavirus from ticks collected on the surface of domestic animals. Bing Du Xue Bao. 2012; 28:252–257.
5. Jiao Y, Zeng X, Guo X, Qi X, Zhang X, Shi Z, Zhou M, Bao C, Zhang W, Xu Y, Wang H. Preparation and evaluation of recombinant severe fever with thrombocytopenia syndrome virus nucleocapsid protein for detection of total antibodies in human and animal sera by double-antigen sandwich enzyme-linked immunosorbent assay. J Clin Microbiol. 2012; 50:372–377.

6. Kim KH, Yi J, Kim G, Choi SJ, Jun KI, Kim NH, Choe PG, Kim NJ, Lee JK, Oh MD. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg Infect Dis. 2013; 19:1892–1894.

7. Li Z, Qi X, Zhou M, Bao C, Hu J, Wu B, Wang S, Tan Z, Fu J, Shan J, Zhu Y, Tang F. A two-tube multiplex real-time RT-PCR assay for the detection of four hemorrhagic fever viruses: severe fever with thrombocytopenia syndrome virus, Hantaan virus, Seoul virus, and dengue virus. Arch Virol. 2013; 158:1857–1863.

8. Libeau G, Diallo A, Calvez D, Lefèvre PC. A competitive ELISA using anti-N monoclonal antibodies for specific detection of rinderpest antibodies in cattle and small ruminants. Vet Microbiol. 1992; 31:147–160.

9. Liu JW, Wen HL, Fang LZ, Zhang ZT, He ST, Xue ZF, Ma DQ, Zhang XS, Wang T, Yu H, Zhang Y, Zhao L, Yu XJ. Prevalence of SFTSV among Asian house shrews and rodents, China, January-August 2013. Emerg Infect Dis. 2014; 20:2126–2128.

10. Magurano F, Nicoletti L. Humoral response in Toscana virus acute neurologic disease investigated by viral-proteinspecific immunoassays. Clin Diagn Lab Immunol. 1999; 6:55–60.

11. Martín-Folgar R, Lorenzo G, Boshra H, Iglesias J, Mateos F, Borrego B, Brun A. Development and characterization of monoclonal antibodies against Rift Valley fever virus nucleocapsid protein generated by DNA immunization. MAbs. 2010; 2:275–284.

12. Park SW, Han MG, Yun SM, Park C, Lee WJ, Ryou J. Severe fever with thrombocytopenia syndrome virus, South Korea, 2013. Emerg Infect Dis. 2014; 20:1880–1882.

13. Park SW, Song BG, Shin EH, Yun SM, Han MG, Park MY, Park C, Ryou J. Prevalence of severe fever with thrombocytopenia syndrome virus in Haemaphysalis longicornis ticks in South Korea. Ticks Tick Borne Dis. 2014; 5:975–977.

14. Spitzer M, Wildenhain J, Rappsilber J, Tyers M. BoxPlot: a web tool for generation of box plots. Nat Methods. 2014; 11:121–122.
15. Sun JM, Zhang YJ, Gong ZY, Zhang L, Lv HK, Lin JF, Chai CL, Ling F, Liu SL, Gu SP, Zhu ZH, Zheng XH, Lan YQ, Ding F, Huang WZ, Xu JR, Chen EF, Jiang JM. Seroprevalence of severe fever with thrombocytopenia syndrome virus in southeastern China and analysis of risk factors. Epidemiol Infect. 2015; 143:851–856.

16. Sun Y, Liang M, Qu J, Jin C, Zhang Q, Li J, Jiang X, Wang Q, Lu J, Gu W, Zhang S, Li C, Wang X, Zhan F, Yao W, Bi Z, Wang S, Li D. Early diagnosis of novel SFTS bunyavirus infection by quantitative real-time RT-PCR assay. J Clin Virol. 2012; 53:48–53.

17. Takahashi T, Maeda K, Suzuki T, Ishido A, Shigeoka T, Tominaga T, Kamei T, Honda M, Ninomiya D, Sakai T, Senba T, Kaneyuki S, Sakaguchi S, Satoh A, Hosokawa T, Kawabe Y, Kurihara S, Izumikawa K, Kohno S, Azuma T, Suemori K, Yasukawa M, Mizutani T, Omatsu T, Katayama Y, Miyahara M, Ijuin M, Doi K, Okuda M, Umeki K, Saito T, Fukushima K, Nakajima K, Yoshikawa T, Tani H, Fukushi S, Fukuma A, Ogata M, Shimojima M, Nakajima N, Nagata N, Katano H, Fukumoto H, Sato Y, Hasegawa H, Yamagishi T, Oishi K, Kurane I, Morikawa S, Saijo M. The first identification and retrospective study of Severe Fever with Thrombocytopenia syndrome in Japan. J Infect Dis. 2014; 209:816–827.

18. Taylor LH, Latham SM, Woolhouse ME. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001; 356:983–989.

19. Woolhouse MEJ, Taylor LH, Haydon DT. Population biology of multihost pathogens. Science. 2001; 292:1109–1112.

20. Wu W, Zhang S, Qu J, Zhang Q, Li C, Li J, Jin C, Liang M, Li D. Simultaneous detection of IgG antibodies associated with viral hemorrhagic fever by a multiplexed Luminex-based immunoassay. Virus Res. 2014; 187:84–90.

21. Xu B, Liu L, Huang X, Ma H, Zhang Y, Du Y, Wang P, Tang X, Wang H, Kang K, Zhang S, Zhao G, Wu W, Yang Y, Chen H, Mu F, Chen W. Metagenomic analysis of fever, thrombocytopenia and leukopenia syndrome (FTLS) in Henan province, China: discovery of a new bunyavirus. PLoS Pathog. 2011; 7:e1002369.

22. Yathi KK, Bhasker S, Chinnamma M. Determination of B cell epitopes and evaluation of antigen capture ELISA for the earlier diagnosis of CHIK virus using anti-rCHIK E1 rabbit antibodies. J Immunol Methods. 2013; 393:45–52.

23. Yu L, Zhang L, Sun L, Lu J, Wu W, Li C, Zhang Q, Zhang F, Jin C, Wang X, Bi Z, Li D, Liang M. Critical epitopes in the nucleocapsid protein of SFTS virus recognized by a panel of SFTS patients derived human monoclonal antibodies. PLoS One. 2012; 7:e38291.

24. Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, Jing HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, Ren J, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011; 364:1523–1532.

25. Yun SM, Lee WG, Ryou J, Yang SC, Park SW, Roh JY, Lee YJ, Park C, Han MG. Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013. Emerg Infect Dis. 2014; 20:1358–1361.

Supplementary Material
Supplementary data is available at http://www.vetsci.org only.
Supplementary Fig. 1
Determination of optimized working concentration of the antigen and HRP-conjugated mAb in cELISA. (A) Influence of antigen concentrations and dilution rates of NP-derived polyclonal antibody on the percent inhibition rates were measured using cELISA. cELISA was performed using plates coated with concentrations of antigen ranging from 50 to 200 ng/well, and using different dilutions of polyclonal antibodies. (B) Influence of the concentrations of HRP-conjugated mAb and the dilution rate of NP-derived polyclonal antibody.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download