Abstract
This report describes the usefulness of positron emission tomography-computed tomography (PET-CT) for evaluating recurrent or residual tumors following surgery. CT and 18F-fluorodeoxyglucose PET-CT were pre- and post-operatively applied to multiple masses in a dog with hemangiosarcoma. The distinction between the left subcutaneous mass and the peritoneum was clarified on pre-operative CT examination, and malignancy was suspected based on PET-CT. A recurrent or residual tumor in the left subcutaneous region was suspected on post-operative PET-CT, and confirmed through histopathologic examination.
Hemangiosarcoma has the potential to affect any tissue in the body, with the three most common primary sites being the spleen (28–50%), right atrium and auricle (3–50%), and skin or subcutaneous tissue (13%) [4]. Metastases to the liver, lungs, and heart are common. It is often difficult to determine the primary tumor site in dogs with advanced hemangiosarcoma if the lesions are disseminated [5]. About 80% of dogs with hemangiosarcomas have metastases at the time of original diagnosis [7]. It is important to define tumor extension and detect local or distant metastases to plan appropriate management or surgery.
Pre-operative diagnostic imaging usually evaluates tumors with regard to morphology, size, location, extent, and presence of secondary complications, and determines the regional or distant metastasis. Positron emission tomography-computed tomography (PET-CT) is considered to be one of the most sensitive diagnostic modalities for evaluating tumor stage, response to therapy, and recurrence [136]. 18F-fluorodeoxyglucose (18F-FDG) uptake provides useful information during follow-up PET-CT examination; specifically, if metabolic activity significantly decreases in a malignant lesion, it can be considered a good response to therapy. Conversely, recurrence may be considered if metabolic activity increases or develops de novo in a pre-existing lesion.
In this study, we described the application of CT and PET-CT for identification of residual tumors following surgical excision of multiple hemangiosarcoma sites of the subcutaneous, intra-muscular, and caudal abdomen regions.
A 10-year-old female Maltese presented with a reddish projecting perianal mass, approximately 1 cm in size, below the anus. Another firm mass, approximately 3 cm in size, was palpated in the subcutaneous region of the left caudal thorax. Only mild leukocytosis (21.37 K/µL; reference range, 5.05–16.76 K/µL) was found upon complete blood count. Serum biochemistry and electrolytes were within reference range. Neither mass nor lung metastasis was identified on radiography of the thorax and abdomen. The perianal mass was hypoechoic on ultrasonography and measured 0.9 × 0.5 cm in size, with definitive margins. The subcutaneous mass was 3.7 × 2.1 cm, and had mixed echo patterns with minimal blood flow. Although a part of the adjacent abdominal wall was separately observed, the abdominal wall was suspected to be involved in the mass because ribs were observed within the mass.
CT images revealed the left cranial mass showing homogeneous soft-tissue attenuation within the subcutaneous region. The mass displaced the left cranial peritoneum and left hepatic lobes inward, but there was a distinction between the mass and the peritoneum, and no invasion of the abdominal cavity (Fig. 1). The mass involved the left 11th rib. The costal cartilage of the left 11th rib was lost, but bone structure was preserved without change. The mass was heterogeneously enhanced, with distinct margins in the arterial phase, and contrast enhancement of the mass was persistent and increased in the venous phase following intravenous injection of 880 mg iodine/kg iohexol (Omnipaque; GE Healthcare, China) at a rate of 3 mL/s. The perianal mass showed heterogeneous attenuation with distinct margins and marked contrast enhancement. Two other masses showing similar density and contrast patterns to those of the two masses described previously were found at the left infraspinatus muscle (1.1 × 0.8 cm) and at the caudal abdomen to the right of the urinary bladder (11 × 8.1 mm). In addition, an enlarged sternal lymph node showed homogeneous contrast enhancement.
A staging workup was performed using PET-CT (Discovery 600 PET-CT system; GE Healthcare) at 50 min after intravenous injection of 11 MBq/kg 18F-FDG. There were multiple hypermetabolic lesions, which were seen in the left subcutaneous, perianal, and right abdominal areas, as well as the diaphragmatic parietal pleura, left infraspinatus muscle, and sternal lymph node (Fig. 2). The metabolic activity of each lesion represented as maximum standardized uptake values (SUVmax) ranged from 2.1 to 6.2 (Table 1).
On cytologic examination, the subcutaneous mass had relatively low cellularity and was composed of spindle cells and damaged cells suspected to be small lymphocytes, with amorphous cytoplasm and eosinophilic substances. Chronic inflammation due to lymphatic duct obstruction was tentatively diagnosed.
Because it was difficult to determine if one of the masses was a primary tumor and the others metastases, and because chronic inflammation was suspected from the cytologic examination, surgical removal of the masses was planned as therapy, as well as for definitive diagnosis. The perianal, left subcutaneous, and right abdominal masses were surgically resected; however, the mass in the left infraspinatus muscle was too small to be removed. The left subcutaneous mass had not invaded adjacent organs, but had firmly adhered to the internal intercostal muscle involving the 11th rib. The mass was removed as completely as possible, and the other two masses were removed without problems. All three masses were diagnosed as hemangiosarcoma on histological examination. No chemotherapy was administered, as the owner did not consent to additional therapy.
One month later, the removal site of the left subcutaneous mass, hemangiosarcoma, was firm and swollen, and protruded slightly. On CT, the swollen left subcutaneous lesion measured 3.7 × 2.1 cm in size, and showed a similar density and contrast pattern to pre-operative images, although the degree of contrast enhancement had decreased. There was no remarkable CT finding from the perianal or right caudal abdomen regions. The size and density of the mass in the left infraspinatus muscle had not changed. The sternal lymph node had diminished in size, and had a lower enhancement pattern than in pre-operative images. Multiple pulmonary nodules, approximately 3 to 4 mm in diameter, were also identified in the right cranial and caudal lung lobes, and in the left cranial lobe. Focal bone thickening and fracture were observed in the left 8th rib, and the right 4th and 5th ribs.
On PET-CT, significant hypermetabolism was no longer present in the right abdominal area. However, a somewhat focal hypermetabolic lesion was still observed in the operative bed of the left subcutaneous area, and metabolic activity was increased in the diaphragmatic parietal pleura. Additionally, a focal hypermetabolic lesion was present in the right lung. On the other hand, the hypermetabolic lesion had disappeared from the sternal lymph node. In addition, hypermetabolic activity was still found around the rectum, but the metabolic pattern was linear along the bowel loop, contrary to the pre-operative PET-CT.
Because of the poor prognosis, the dog was euthanized and necropsied. Tumor recurrence was distinctly observed in the subcutaneous and diaphragmatic parietal pleura regions, anatomically close to the previously removed left subcutaneous mass, and involving internal intercostal muscles with multiple disseminated nodules 0.4 to 0.5 cm size. Metastatic lesions including a mass 0.5 cm size in the omentum and multiple pulmonary masses were found. Left infraspinatus muscle and sternal lymph node showed no remarkable findings on necropsy.
Hemangiosarcomas are generally surrounded by a pseudocapsule with poorly defined margins, and infiltration of the surrounding tissues. Therefore, although the tumor may appear to be easily extirpated by conservative surgery, there is usually a high rate of recurrence due to infiltrated margins [8]. In this case, we considered an anatomic distinction, without invasion, to be present between the left subcutaneous mass and the peritoneum on the pre-operative CT images, but a hypermetabolic signal was observed from the diaphragmatic parietal pleura region and from the subcutaneous mass on PET-CT. The left subcutaneous lesion persistently showed focal hypermetabolism on the post-operative PET-CT, which suggested a residual tumor. However, post-operative change could not be ruled out because surgery-related inflammation can affect 18F-FDG uptake for up to 6 months [9]. 18F-FDG de novo studies should be carried out within the first 2 days post-surgery or following a delay of at least 3 to 4 weeks after surgery to allow surgery-related inflammation to subside, and the presence of residual tumor to be accurately interpreted. Post-operative PET-CT of the present case was performed at 60 days after surgery. The mass-like lesion with focal 18F-FDG uptake in the site of the left subcutaneous lesion favored a diagnosis of recurrence and/or residual lesion, rather than an inflammatory lesion. Metabolic activity in the right lung on the post-operative PET-CT suggested an aggravated malignancy or newly developed metastasis. The sternal lymph node was considered to be a reactive response rather than metastasis because hypermetabolic activity had disappeared after surgery. In addition, hypermetabolism in the perianal area was considered a post-operative change or physiologic bowel uptake rather than residual malignancy, as the metabolic pattern was linear along the bowel loop.
A SUVmax of 2.0 was defined as the threshold value for glucose metabolism in human bone or soft-tissue tumors, with a maximum sensitivity of 97.7% and a specificity of 100% [2]. All of the masses identified in our case could be presumed to be malignancies based on the criteria of SUVmax, although canine studies have not yet been performed. However, when the mass size is small, a malignancy cannot be ruled out, despite low 18F-FDG uptake. This is because metabolic activity may be underestimated in small-sized lesions. Lesion detectability is largely dependent on location and motion. Lesion detectability is increased in areas with minimal motion and minimal background physiologic activity. In our case, the right lung nodule was newly identified, but had very low uptake. In the lungs, background activity is low, but respiratory motion decreases detection. After taking the patient's history and other lesions into account, it was suspected as a metastasis.
This study describes the diagnostic features of pre- and post-operative CT and PET-CT for canine hemangiosarcoma. Whole-body PET-CT is particularly valuable for investigating malignant potential, identifying multiple masses, and determining residual or recurrent tumors; therefore, it is indispensable for identifying distant metastases.
Figures and Tables
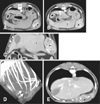 | Fig. 1Pre- (A–D) and post- (E) operative computed tomography (CT) images. On transverse CT images, the left subcutaneous mass (*) showed heterogeneous enhancement at the arterial phase (A) and the contrast enhancement was persistent and increased at the venous phase (B). On the dorsally reformatted CT image (C), the mass displaced the peritoneum and left hepatic lobes (l) inward, but had smooth margins with no invasion of the abdominal cavity. (D) On the volume rendering image, the costal cartilage of the left 11th rib was lost (arrows), but bone structure was preserved without change. (E) On the post-operative transverse CT image, the left subcutaneous region (*) was swollen, with a similar density and contrast enhancement pattern, compared to the pre-operative CT images. |
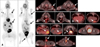 | Fig. 2Positron emission tomography and computed tomography (PET-CT) images using 18F-fluorodeoxyglucose. Pre- (left) and post- (right) operative maximum intensity projection views (A) showed abnormal hypermetabolic lesions (arrows), as well as physiological uptake (especially in the brain, heart, kidneys, urinary bladder, etc.). On each transverse plane of the PET-CT fusion images (B–G), all images were ordered in the cranial to caudal direction with pre- (left) and post- (right) operative images. There were multiple hypermetabolic lesions in the left infraspinatus muscle (B), sternal lymph node (C), diaphragmatic parietal pleura (D), and left subcutaneous (E), right abdominal (F), and perianal (G) areas (arrows). a, brain; b, injection site; c, heart; d, kidney; e, urinary bladder. |
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT, and Future Planning (2015R1A2A2A01003313).
We thank Young-Seok Lee and Hyun-O Oh for technical assistance with the PET-CT examination.
References
1. Al-Ibraheem A, Buck AK, Benz MR, Rudert M, Beer AJ, Mansour A, Pomykala KL, Haller B, Juenger H, Scheidhauer K, Schwaiger M, Herrmann K. 18F-fluorodeoxyglucose positron emission tomography/computed tomography for the detection of recurrent bone and soft tissue sarcoma. Cancer. 2013; 119:1227–1234.

2. Bischoff M, Bischoff G, Buck A, von Baer A, Pauls S, Scheffold F, Schultheiss M, Gebhard F, Reske SN. Integrated FDG-PET-CT: its role in the assessment of bone and soft tissue tumors. Arch Orthop Trauma Surg. 2010; 130:819–827.

3. Cheon GJ, Kim MS, Lee JA, Lee SY, Cho WH, Song WS, Koh JS, Yoo JY, Oh DH, Shin DS, Jeon DG. Prediction model of chemotherapy response in osteosarcoma by 18F-FDG PET and MRI. J Nucl Med. 2009; 50:1435–1440.

4. Clifford CA, Mackin AJ, Henry CJ. Treatment of canine hemangiosarcoma: 2000 and beyond. J Vet Intern Med. 2000; 14:479–485.

5. Hargis AM, Ihrke PJ, Spangler WL, Stannard AA. A retrospective clinicopathologic study of 212 dogs with cutaneous hemangiomas and hemangiosarcomas. Vet Pathol. 1992; 29:316–328.

6. Herrmann K, Bundschuh RA, Rosenberg R, Schmidt S, Praus C, Souvatzoglou M, Becker K, Schuster T, Essler M, Wieder HA, Friess H, Ziegler SI, Schwaiger M, Krause BJ. Comparison of different SUV-based methods for response prediction to neoadjuvant radiochemotherapy in locally advanced rectal cancer by FDG-PET and MRI. Mol Imaging Biol. 2011; 13:1011–1019.

7. MacEwen EG. Spontaneous tumors in dogs and cats: models for the study of cancer biology and treatment. Cancer Metastasis Rev. 1990; 9:125–136.

8. MacEwen EG, Powers BE, Macy D, Withrow SJ. Soft tissue sarcomas. In : Withrow SJ, MacEwen EG, editors. Small Animal Clinical Oncology. Philadelphia: WB Saunders;2001. p. 283–304.
9. Schelbert HR, Hoh CK, Royal HD, Brown M, Dahlbom MN, Dehdashti F, Wahl RL. Society of Nuclear Medicine Procedure Guideline for Tumor Imaging Using F-18 FDG. Ver. 2.0, approved February 7, 1999. Retired Procedure Standards. Reston: Society of Nuclear Medicine and Molecular Imaging;1999.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download