Abstract
Caudal pulmonary artery diameter (CPAD) to body surface area (BSA) ratios were measured in ventrodorsal thoracic radiographs to assess the correlation between CPAD to BSA ratios and systolic pulmonary arterial pressure (PAP) in dogs. Thoracic radiographs of 44 dogs with systolic pulmonary arterial hypertension (PAH) and 55 normal dogs were evaluated. Systolic PAP was estimated by Doppler echocardiography. CPADs were measured at their largest point at the level of tracheal bifurcation on ventrodorsal radiographs. Both right and left CPAD to BSA ratios were significantly higher in the PAH group than in the normal group (p < 0.0001). Linear regression analysis showed positive associations between PAP and right and left CPAD to BSA ratio (right, p = 0.0230; left, p = 0.0012). The receiver operating characteristic curve analysis revealed that the CPAD to BSA ratio had moderate diagnostic accuracy for detecting PAH. The operating point, sensitivity, specificity, and area under the curve were 28.35, 81.40%, 81.82%, and 0.870; respectively, for the right side and 26.92, 80.00%, 66.67%, and 0.822, respectively, for the left. The significant correlation of CPAD to BSA ratio with echocardiography-estimated systolic PAP supports its use in identifying PAH on survey thoracic radiographs in dogs.
Pulmonary hypertension refers to the dynamic state in which the pressure measured in the pulmonary artery is elevated. In normal dogs, the normal mean pulmonary arterial pressure (PAP) ranges from 10 to 15 mmHg, and the systolic PAP ranges from 15 to 25 mmHg [15]. Pulmonary hypertension is regarded as a systolic PAP greater than approximately 30 mmHg [11214]. PAP is influenced by pulmonary blood flow, pulmonary vascular resistance, and pulmonary venous pressure. Elevated PAP may be caused by pulmonary vascular abnormalities associated with increased blood flow, changes affecting pulmonary vascular resistance to flow (pre-capillary pulmonary arterial hypertension), or increased resistance (post-capillary pulmonary venous hypertension) [13].
Pulmonary hypertension in dogs can be primary or secondary to various diseases, including pulmonary venous and left atrial hypertension (e.g., degenerative mitral valve disease and left-sided congestive heart failure), pulmonary thromboembolic disease (e.g., dirofilariasis, Cushing's syndrome, protein losing nephropathy, or enteropathy), congenital cardiac abnormalities (e.g., reversed patent ductus arteriosus), and chronic pulmonary disease (e.g., chronic bronchitis or pulmonary fibrosis) [111]. The underlying causes of pulmonary hypertension are not readily reversible, and prognosis of severe pulmonary hypertension is poor in both human and veterinary medicine [1214]. Therefore, early recognition of the pulmonary hypertension is essential in veterinary clinics.
Cardiac catheterization is the gold standard for diagnosis of pulmonary arterial hypertension (PAH); however, it is rarely performed in veterinary medicine because of the invasive nature of the procedure and the requirement for general anesthesia. Sedation or general anesthesia may alter the accuracy of pressure measurement [15]. Noninvasive prediction of PAP is possible with Doppler echocardiography using the modified Bernoulli equation applied to the peak velocity of tricuspid regurgitation or pulmonary regurtitant flow [19]. Previous studies in human medicine revealed good agreement between Doppler-estimated and catheterization-measured systolic PAP, and this method has been validated and confirmed to be reliable in the past few decades [1924]. Thoracic radiography is believed to be nonspecific for diagnosis of pulmonary hypertension and unable to detect pathognomonic changes [712]. However, identification of underlying disease is important to diagnosis of pulmonary hypertension and to estimate the patient's prognosis. One study reported that thoracic radiographs were abnormal in 98% of dogs with pulmonary hypertension and the following radiographic findings were reported: cardiomegaly (84%), pulmonary parenchymal infiltration (65%), and enlarged pulmonary arteries (31%) [12].
In human medicine, many attempts have been made to correlate pulmonary artery size determined using various imaging techniques with PAP measured via catheterization of the right side of the heart [10]. Several studies have shown that the right descending pulmonary artery diameter on chest radiographs was significantly correlated with echocardiography-estimated systolic PAP [5671618]. However, no studies in veterinary medicine have been conducted for quantitative assessment of pulmonary artery size associated with PAH in thoracic radiography.
Therefore, this study was conducted to assess the correlation between caudal pulmonary artery diameter (CPAD) to body surface area (BSA) ratios and systolic PAH in dogs. Furthermore, optimal cut-off points of CPAD to BSA ratios were investigated to assess the clinical relevance of this value for identifying PAH in dogs relative to the broadly used method of the pulmonary artery diameter crossing the rib in thoracic radiographs.
This retrospective study used medical and diagnostic imaging records from February 2009 to September 2012 from the Veterinary Medical Center, Chungbuk National University. Inclusion criteria for dogs with pulmonary hypertension included: (1) a definitive diagnosis of PAH based on clinical signs, physical examination, thoracic radiography, and echocardiography; (2) PAP > 30 mmHg estimated by Doppler echocardiography; and (3) available right lateral, ventrodorsal thoracic radiographs taken within 24 h of the echocardiography examinations.
A total of 53 dogs with PAH met the above criteria. Among these dogs, 44 were finally selected for inclusion in the study, while 9 were excluded because of incomplete medical records (body weight, age). Additionally, 55 dogs diagnosed as having a normal thorax were randomly selected from the radiology log for comparison during the same period. Medical records from the control dogs were screened to ensure the absence of cardiovascular or pulmonary diseases on thoracic radiography and echocardiography. All dogs were negative for canine heartworm based on the results of an antigen test kit.
All dogs with PAH had detectable tricuspid regurgitation on Doppler echocardiography; hence, it was possible to estimate systolic PAP. Echocardiograms were obtained using 1 of 2 ultrasound machines (ProSound Alpha 5SV or Alpha 7; Hitachi Aloka Medical, Japan), and no dogs were sedated for examination. Complete 2-dimensional and Doppler echocardiographic studies were performed. Pulmonic stenosis was ruled out in all dogs based on lack of abnormality of the right ventricular outflow tract and normal pulmonary arterial flow on Doppler echocardiography. The continuous-wave Doppler detected the flow of tricuspid regurgitation. The tricuspid regurgitation pressure gradient (ΔP) was calculated from its peak velocity (V) according to the modified Bernoulli equation (ΔP = 4 × V2) based on more than three measurements. Systolic PAP was estimated by adding the tricuspid regurgitation pressure gradient to the right atrial pressure. If the atrial septum had curvature to the left side of the heart, the right atrium was considered dilated. Right atrial pressure was estimated to be: (1) 5 mmHg when there was no evidence of right atrial dilation, (2) 10 mmHg if there was right atrial dilation with no evidence of right-sided congestive heart failure, and (3) 15 mmHg when there was right-sided congestive heart failure with right atrial dilation [15]. Dogs with PAH were categorized as having mild PAH (systolic PAP < 50 mmHg), moderate PAH (51–75 mmHg), and severe PAH (> 75 mmHg) [22].
From the thoracic radiographs of all 99 dogs, the following measurements were made by 2 blinded observers (Choi and D. Lee) using the DICOM viewing software (eFilm Workstation 3.0.1; Merge Healthcare, USA). The maximum right and left CPADs at the level of the tracheal bifurcation were measured on ventrodorsal thoracic radiographs (Fig. 1). If the margin of the caudal pulmonary artery was not demarcated, as agreed by two observers (Choi and D. Lee), they were excluded from the measurements. Measured CPAD was adjusted for BSA (body weight in kilograms2/3 × 0.101) in square meters [4] and converted to CPAD to BSA ratios to calibrate body size of the dogs.
To compare the pulmonary artery diameter with rib widths, right lateral and ventrodorsal thoracic radiographs were reviewed. The summation shadow of the intersecting pulmonary artery and the rib at the point where the vessels cross the 4th or 9th rib was also evaluated [24]. If the shadow width was larger than the rib width, the pulmonary artery was considered to be enlarged, provided two observers reached a consensus.
All statistical analyses were carried out with commercially available software (GraphPad Prism 6; GraphPad Software, USA). Numerical values were presented as the mean ± standard deviation. The difference in CPAD to BSA ratio between dogs with and without PAH was assessed by the Mann-Whitney U test. The difference of the CPAD to BSA ratio between groups according to the severity of PAH was assessed by the Kruskal-Wallis test, and post hoc analysis was conducted by Dunn's multiple comparison test. Linear regression analysis was performed to assess the relationship between systolic PAP and right and left CPAD to BSA in dogs with PAH. Receiver operating characteristic curves, visual inspection of box and whisker plots, and likelihood ratio tables were used to identify the optimal cut-off points that maximized both sensitivity and specificity in diagnosing PAH. The area under the receiver operating characteristic curve was calculated to determine the accuracy. The area under the curve was used as the following scales: excellent, 0.90–1.0; good, 0.80–0.90; fair, 0.70–0.80; poor, 0.60–0.70; and fail, 0.50–0.60. A p < 0.05 was considered statistically significant.
The characteristics of the dogs with and without PAH are summarized in Table 1. There were 55 dogs in the normal group and 44 dogs in the PAH group. Dogs with PAH were categorized according to the severity of PAH. Overall, there were nine dogs in the mild PAH group (systolic PAP < 50 mmHg), 13 dogs in the moderate PAH group (systolic PAP, 51–75 mmHg), and 22 dogs in the severe PAH group (systolic PAP > 75 mmHg). The mean ages (years) of dogs with and without PAH were 10.89 ± 2.88 and 9.36 ± 3.49, respectively, and the mean body weights (kg) were 5.93 ± 5.97 and 5.70 ± 2.84, respectively. There was no statistical difference in age or body weight between dogs with and without PAH. In the dogs with PAH, there was a tendency for increasing age and decreasing body weight with increasing severity of PAH; however, this difference was not statistically significant. Different breeds were included in similar proportions in both groups.
In dogs with PAH, the underlying cause was identified as either chronic valvular heart disease with mitral regurgitation and left-sided congestive heart failure (n = 27, 61.36%), chronic pulmonary disease (n = 10, 22.72%), dirofilariasis (n = 7, 15.90%), or suspected pulmonary thromboembolism due to malignant lymphoma (n = 1, 2.27%). Both chronic valvular heart disease and dirofilariasis were diagnosed in one of the dogs.
The left and right CPAD to BSA ratios were 23.87 ± 7.02 and 24.02 ± 5.70, respectively, in the normal group and 31.70 ± 6.72 and 34.40 ± 7.40, respectively, in the PAH group (Table 2). Based on the Mann-Whitney U test, the left and right CPAD to BSA ratios were significantly higher in the PAH group than in the normal group (p < 0.0001) (Fig. 2), while the left and right CPAD to BSA ratios were significantly higher in the moderate and severe PAH groups than in the normal group (Fig. 3).
To elucidate the relationship between systolic PAP and the left and right CPAD to BSA ratios, scatter plots of systolic PAP and both the CPAD to BSA ratios were drawn, and predicted linear regression lines were developed. Linear regression analysis showed positive associations between systolic PAP and both left and right CPAD to BSA ratios in the PAH group (p = 0.0012, p = 0.0230, respectively). The results revealed that the systolic PAP = left CPAD to BSA ratio × 2.1421 + 12.566 and systolic PAP = right CPAD to BSA ratio × 1.6021 + 27.218. Goodness of fits were low in both left and right CPAD to BSA ratios (R2 = 0.2447, R2 = 0.1197, respectively) (Fig. 4).
On the receiver operating characteristic curves of the left and right CPAD to BSA ratios, the operating points to simultaneously maximize sensitivity and specificity were 26.92 and 28.35, respectively. At each operating point, the sensitivity and specificity were 80.00 and 66.67 on the left side and 81.40 and 81.82 on the right side. The sensitivity, specificity, and likelihood ratio for the CPAD to BSA ratios are listed in Table 3. The area under the curve values of the left and right CPAD to BSA ratios were 0.822 and 0.870, respectively (p < 0.0001). The left and right CPAD to BSA ratios had good diagnostic accuracies (0.80–0.90) for detecting PAH (Fig. 5).
In 99 thoracic radiographs of dogs with and without PAH, only one (1.01%) right caudal pulmonary artery was not clearly visible, while 7 (7.07%) left caudal pulmonary arteries were not clearly visible. This difficulty in visualizing the caudal pulmonary arteries was typically caused by superimposition of the left side of the heart, the mediastinum, or pulmonary infiltration.
When CPAD was assessed by comparison with rib widths, enlarged pulmonary arteries were shown in seven dogs (17.50%) on the right lateral thoracic radiographs, and 28 dogs (63.63%) on ventrodorsal thoracic radiographs in the PAH group. The confusion matrix, sensitivity, specificity, and overall accuracy for pulmonary artery comparison with rib width are listed in Table 4. The overall accuracies in comparison with the 4th and 9th rib width were 0.631 and 0.747, respectively. The sensitivity and accuracy values from comparison with rib width were lower than those achieved using the left and right CPAD to BSA ratios.
The present study demonstrated that the CPAD to BSA ratio derived from thoracic radiographs was significantly correlated with echocardiography-estimated systolic PAP in dogs. However, estimation of systolic PAP by linear regression equation with the CPAD to BSA ratio was difficult because goodness of fits were low in both left and right CPAD to BSA ratios (R2 = 0.2447, R2 = 0.1197, respectively). Both left and right CPAD to BSA ratios provided a good level of accuracy for predicting the presence of PAH, and the sensitivity and specificity at operating points were favorable. Overall, the results of the present study show that the left and right CPAD to BSA ratios are useful and clinically valid measurements in dogs with PAH.
In human medical literature, numerous studies have suggested a correlation between catheter- or echocardiography-derived PAP and the right descending pulmonary artery diameter on chest radiography. However, the cut-off points of right descending pulmonary artery diameter differed for each technique (16 mm–20 mm), and sensitivity, specificity, and accuracy were also variable [567101618]. In comparison, several studies that used the hilar thoracic ratio instead of absolute right descending pulmonary artery diameter values showed higher sensitivity and specificity [817]. The hilar thoracic ratio is a transhilar diameter divided transverse thoracic diameter on anterior-posterior radiographs in human medicine [17]. However, the hilar thoracic ratio was not used in the present study because of the diverse thoracic conformations of the different breeds of dogs. In this study, BSA was used to compensate for variations in dog size. The method of CPAD measurement was borrowed in human reports [5671618]. Similar to the right descending pulmonary artery in human reports, right CPAD to BSA ratio had higher sensitivity, specificity, and accuracy to differentiate PAH in dogs than left CPAD to BSA ratio. This may be because the left pulmonary artery is shorter and slightly smaller in diameter than the right pulmonary artery in dogs [2]. In addition, the left caudal pulmonary artery may be obscured by an enlarged main pulmonary artery or left atrium on ventrodorsal thoracic radiographs.
There are several radiographic signs associated with PAH; namely, right sided cardiomegaly, dilation of the main pulmonary artery, or pulmonary artery intersecting the 4th or 9th ribs [2021]. However, quantitative evaluation of the enlarged right heart and the dilated main pulmonary artery was unreliable. Right atrial dilation was subjectively assessed and the assessment depended on the observer's experience [22]. As a result, only comparison between the pulmonary artery and rib width was used as a comparison to CPAD to BSA ratio in this study. The overall accuracy of the rib width comparison method (0.631, 0.747) was lower than that of the CPAD to BSA ratios (0.822, 0.870) and the sensitivity of this method was very low (17.50%), suggesting that this is not an appropriate diagnostic tool for screening PAH.
Based on the CPAD to BSA ratio, dogs with PAH had larger CPAD than normal dogs. A high CPAD to BSA ratio may reflect an enlarged caudal pulmonary artery as well as a small BSA. As a result, a dog with a low body condition score might have a higher CPAD to BSA ratio without necessarily having increased CPAD. However, it was not possible to investigate this further as body condition score was not always available in this retrospective study.
There was no difference in the CPAD to BSA ratios of the mild PAH group and normal group. However, there are still methodological issues that should be taken into consideration. For example, poorly detectable tricuspid regurgitation makes the assessment of spectral contour difficult. Additionally, the modified Bernoulli equation usually overestimates the pressure gradient to a small degree because it ignores the contribution of flow acceleration and viscous friction [12]. Accordingly, the systolic PAP of dogs with mild PAH (30–50 mmHg) might have been overestimated in this study.
As reported in a previous study, chronic valvular heart disease with mitral regurgitation was a major cause of PAH (61.36%) [11], while chronic pulmonary disease with alveolar hypoxia and dirofilariasis with vascular obstruction were the second and third main causes, respectively. One unclassified dog had a history of a splenic mass diagnosed as lymphoma on histopathology. However, no necropsy data were available for this dog to identify potential causes for increased PAP. Additionally, there was no difference in the pathophysiologic classification of primary or secondary diseases based on the CPAD to BSA ratios of the 3 groups.
Receiver operating characteristic curves and likelihood ratios illustrate the potential usefulness of certain cut-off points for the CPAD to BSA ratios evaluated in this study. The receiver operating characteristic curve provides more information about how the test performs than a single estimate of sensitivity and specificity. The closest point to the upper left hand corner on the curve is the operating point that minimizes the sum of false positive and false negative results [23]. The operating points of the CPAD to BSA ratios were 26.92 (left) and 28.35 (right) in this study.
Likelihood ratio tables for both left and right CPAD to BSA ratios express the odds that a given ratio would be expected in a dog with PAH. Likelihood ratio is less affected by disease prevalence than by sensitivity and specificity. A likelihood ratio > 1 indicates that the test result is associated with the presence of the disease, whereas a likelihood ratio < 1 indicates that the result is associated with absence of the disease [9]. As shown in Table 3, sensitivities at both cut-off points were similar; however, the likelihood ratio of the right CPAD to BSA ratio was about 2 times higher than the left one.
The measurements of CPAD were made based on the ventrodorsal view. However, caudal pulmonary vessels are usually better observed in dorsoventral view than in ventrodorsal view because better lung inflation is achieved in sternal recumbency [21]. However, since few dogs with pulmonary hypertension had the dorsoventral view evaluated upon radiographic examination, those evaluations were not included in this retrospective study.
It should be noted that this study had several limitations. Specifically, although it only affected a small proportion of the images, superimposition of the caudal pulmonary artery did make assessment of CPAD difficult in some cases. Among 99 thoracic radiographs of all dogs, the right caudal pulmonary artery was not clearly visible on only 1%; however, the visibility of about 7% of the left caudal pulmonary arteries was impaired by superimposition with the mediastinum, pulmonary infiltration, or the left side of the heart. In dogs with PAH, pulmonary infiltration was a major cause of superimposition that made it difficult to estimate CPAD.
Therapeutic data, which may have affected the results or the interpretation of findings, was not included in this study. One retrospective study demonstrated that the pressure gradient of right ventricle to right atrium decreased significantly after sildenafil therapy [1]; however, this was not associated with a significant decrease in peak tricuspid regurgitation flow gradient in two other studies [314]. In this study, the majority of dogs had chronic valvular heart disease (61.36%), and the timing of diuretics administration relative to the timing of the radiographs may have influenced vessel size.
Despite a statistically significant difference between groups, the overlapping results of the normal and PAH groups limits the clinical usefulness of the CPAD to BSA ratio to detect PAH in dogs. To clearly distinguish between normal and abnormal dogs at the extremes of each population, the following cut-off points for these ratios were identified: a left CPAD to BSA ratio < 17.93 and the right CPAD to BSA ratio < 21.65 would indicate a dog without PAH, whereas a left CPAD to BSA ratio > 33.96 and a right CPAD to BSA ratio > 39.56 would be abnormal. It is important to note that these values will only help with a minority of clinical patients as most patients fall outside of these ranges.
In summary, our results suggest that the CPAD to BSA ratios on thoracic radiographs are significantly correlated with echocardiography-estimated systolic PAP in dogs. The CPAD to BSA ratio has good diagnostic accuracy for diagnosis of PAH in dogs, and optimal cut-off points were identified as 26.92 (left) and 28.35 (right). This method has both higher sensitivity and specificity than when CPAD is assessed by comparison with the width of the 4th or 9th ribs. Therefore, calculation of the CPAD to BSA ratio may provide a superior means of identifying systolic PAH on thoracic radiographs in dogs.
Figures and Tables
Fig. 1
Measurement of the left and right caudal pulmonary artery diameter. The maximum diameter (black arrows) of the right and left caudal lobe pulmonary arteries at the level of the first bifurcation of the trachea were measured on ventrodorsal thoracic radiographs. A, caudal pulmonary artery; B, caudal lobe main bronchus; V, caudal pulmonary vein.
Fig. 2
Box and whisker plots of the left (A) and right (B) caudal pulmonary artery diameter (CPAD) to body surface area (BSA) ratios in dogs with and without pulmonary arterial hypertension (PAH). Based on the Mann-Whitney U test, the left and right CPAD to BSA ratios were significantly higher in dogs in the PAH group than in the normal group (p < 0.0001). Box and whisker plots exhibit the entire range of data. The box encompasses the 25th–75th percentiles surrounding the median (indicated by the line within the box) and whiskers represent the upper and lower quartiles. A cross shows the mean value.

Fig. 3
Box and whisker plots of the left (A) and right (B) caudal pulmonary artery diameter (CPAD) to body surface area (BSA) ratios in normal dogs and dogs categorized according to severity of pulmonary arterial hypertension (PAH). Based on Dunn's multiple comparison test, the left and right CPAD to BSA ratios were significantly higher in dogs in the moderate and severe PAH groups than in the normal group. Box and whisker plots exhibit the entire range of data. The box encompasses the 25th–75th percentiles surrounding the median (indicated by the line within the box) and whiskers represent the upper and lower quartiles. A cross shows the mean value.
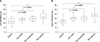
Fig. 4
Correlation between systolic pulmonary arterial pressure (PAP) and left (A) and right (B) caudal pulmonary artery diameter (CPAD) to body surface area (BSA) ratios. Scatter plots for systolic PAP and both CPAD to BSA ratios were drawn, and predicted linear regression lines were developed. Linear regression analysis showed positive associations between systolic PAP and both left and right CPAD to BSA ratios in the pulmonary arterial hypertension group (p = 0.0012, p = 0.0230, respectively). However, goodness of fit was low in both left and right CPAD to BSA ratios (R2 = 0.2447, R2 = 0.1197, respectively).

Fig. 5
Receiver operating characteristic curves of the left (A) and right (B) caudal pulmonary artery diameter (CPAD) to body surface area (BSA) ratio. The left and right areas under the curves were 0.822 and 0.870, respectively. The left and right CPAD to BSA ratios had good diagnostic accuracy for detecting pulmonary arterial hypertension. The area under the curve was used as the following scales: excellent, 0.90–1.0; good, 0.80–0.90; fair, 0.70–0.80; poor, 0.60–0.70; and fail, 0.50–0.60.

Acknowledgments
This work was partially supported by the National Research Foundation of Korea (NRF-2015R1D1A3A01020033).
References
1. Bach JF, Rozanski EA, MacGregor J, Betkowski JM, Rush JE. Retrospective evaluation of sildenafil citrate as a therapy for pulmonary hypertension in dogs. J Vet Intern Med. 2006; 20:1132–1135.

2. Bezuidenhout AJ. The heart and arteries. In : Evans HE, de Lahunta A, editors. Miller's Anatomy of the Dog. 4th ed. Philadelphia: Elsevier Saunders;2013. p. 441.
3. Brown AJ, Davison E, Sleeper MM. Clinical efficacy of sildenafil in treatment of pulmonary arterial hypertension in dogs. J Vet Intern Med. 2010; 24:850–854.

4. Brown DJ, Rush JE, MacGregor J, Ross JN Jr, Brewer B, Rand WM. M-mode echocardiographic ratio indices in normal dogs, cats, and horses: a novel quantitative method. J Vet Intern Med. 2003; 17:653–662.

5. Bush A, Gray H, Denison DM. Diagnosis of pulmonary hypertension from radiographic estimates of pulmonary arterial size. Thorax. 1988; 43:127–131.

6. Chang CH. The normal roentgenographic measurement of the right descending pulmonary artery in 1,085 cases. Am J Roentgenol Radium Ther Nucl Med. 1962; 87:929–935.
7. Chetty KG, Brown SE, Light RW. Identification of pulmonary hypertension in chronic obstructive pulmonary disease from routine chest radiographs. Am Rev Respir Dis. 1982; 126:338–341.
8. Chhabra SK, De S. Clinical significance of hilar thoracic index and width of right descending branch of pulmonary artery in chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci. 2004; 46:91–97.
10. Haimovici JB, Trotman-Dickenson B, Halpern EF, Dec GW, Ginns LC, Shepard JA, McLoud TC. Relationship between pulmonary artery diameter at computed tomography and pulmonary artery pressures at right-sided heart catheterization. Massachusetts General Hospital Lung Transplantation Program. Acad Radiol. 1997; 4:327–334.
12. Johnson L, Boon J, Orton EC. Clinical characteristics of 53 dogs with Doppler-derived evidence of pulmonary hypertension: 1992–1996. J Vet Intern Med. 1999; 13:440–447.

13. Kellihan HB, Stepien RL. Pulmonary hypertension in canine degenerative mitral valve disease. J Vet Cardiol. 2012; 14:149–164.

14. Kellum HB, Stepien RL. Sildenafil citrate therapy in 22 dogs with pulmonary hypertension. J Vet Intern Med. 2007; 21:1258–1264.

15. Kittleson MD, Kienle RD. Pulmonary arterial and systemic arterial hypertension. In : Kittleson MD, Kienle RD, editors. Small Animal Cardiovascular Medicine. Saint Louis: Mosby;1998. p. 433–449.
16. Lin SC, Chen RJC, Lee JH. The correlation between right descending pulmonary artery diameter and echocardiography-estimated systolic pulmonary artery pressure. Zhonghua Minguo Xin Zang Xue Hui Za Zhi. 2009; 25:213–217.
17. Lupi E, Dumont C, Tejada VM, Horwitz S, Galland F. A radiologic index of pulmonary arterial hypertension. Chest. 1975; 68:28–31.

18. Matthay RA, Schwarz MI, Ellis JH Jr, Steele PP, Siebert PE, Durrance JR, Levin DC. Pulmonary artery hypertension in chronic obstructive pulmonary disease: determination by chest radiography. Invest Radiol. 1981; 16:95–100.

19. Nishimura RA, Tajik AJ. Quantitative hemodynamics by Doppler echocardiography: a noninvasive alternative to cardiac catheterization. Prog Cardiovasc Dis. 1994; 36:309–342.

20. Perry LA, Dillon AR, Bowers TL. Pulmonary hypertension in dogs. Compend Contin Educ Pract Vet. 1991; 13:226–232.
21. Robert JB. Heart and pulmonary vessels. In : Thrall DE, editor. Textbook of Veterinary Diagnostic Radiology. 5th ed. Philadelphia: Saunders Elsevier;2007. p. 576–582.
22. Serres FJ, Chetboul V, Tissier R, Sampedrano CC, Gouni V, Nicolle AP, Pouchelon JL. Doppler echocardiographyderived evidence of pulmonary arterial hypertension in dogs with degenerative mitral valve disease: 86 cases (2001–2005). J Am Vet Med Assoc. 2006; 229:1772–1778.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download