Abstract
The Brucella mdh gene was successfully cloned and expressed in E. coli. The purified recombinant malate dehydrogenase protein (rMDH) was reactive to Brucella-positive bovine serum in the early stage, but not reactive in the middle or late stage, and was reactive to Brucella-positive mouse serum in the late stage, but not in the early or middle stage of infection. In addition, rMDH did not react with Brucella-negative bovine or mouse sera. These results suggest that rMDH has the potential for use as a specific antigen in serological diagnosis for early detection of bovine brucellosis.
Brucellosis is a widespread infectious and contagious disease that affects a variety of domestic animals, where it causes abortion and sterility. This disease can also be transmitted to humans, where it is characterized by undulant fever, arthritis and neurological disorders [15]. Since no current safe vaccine is available for human brucellosis, its prevention is mainly dependent on control and prevention in animals, which can be attained through accurate clinical diagnosis and effective vaccination [2]. However, the disease has diverse clinical manifestations with symptoms that are not pathognomonic, and the only test that provides direct evidence for the presence of the pathogen is isolation and identification of the causative agent, which is time-consuming, difficult and dangerous. Moreover, the currently available vaccine for animal brucellosis possesses several drawbacks and cannot eliminate the disease in any host species [4].
Current serological tests are relatively accessible and cheap, more reliable, generally approved and often the preferred method for diagnosis of brucellosis [1]. Despite the strong immunoreactivity of the antigen used in these classical serological tests, which is commonly derived from smooth lipopolysaccharide (LPS) or O-polysaccharide, its specificity is very low and it is often misdiagnosed because of cross-reactions with other clinically relevant Gram-negative bacteria [13]. Conversely, currently available animal vaccines against brucellosis are genetically undefined strains that can induce abortion and persistent infection in animals, as well as in humans [6]. Consequently, there is an increasing interest in identifying more specific Brucella antigen to detect brucellosis, and development of safe and effective Brucella vaccines to control and prevent brucellosis [1116].
In our previous study [7], several immunogenic proteins were identified through two-dimensional electrophoresis followed by immunoblot analysis of sera from experimentally infected mice in the early, middle and late infection periods with the exclusion of cross-reaction with Yersinia enterocolitica O:9-infected and non-infected mice. One of these specific immunogenic proteins is malate dehydrogenase (MDH), which has been detected in the middle stage of infection in mice. The present study is the first attempt to characterize recombinant Brucella (B.) abortus 544 MDH protein (rMDH) expressed in a pMAL vector while focusing on its reactions to hosts during different infection periods. Accordingly, we investigated its immunogenicity using Brucella-positive bovine and mouse sera from three different stages of infection (early, middle and late), which can be used for future studies exploring the potential of rMDH as a serological diagnostic tool and a subunit vaccine candidate.
The standard wild-type strains were derived from B. abortus 544 (ATCC 23448), a smooth, virulent B. abortus biovar 1 strain. The cultivation of B. abortus 544 was conducted in Brucella broth (Becton Dickinson and Company, USA) or Brucella broth containing 1.5% agar (Becton Dickinson and Company) and grown at 37℃. Conversely, cultivation of E. coli DH5α was conducted in Luria-Bertani (LB) broth or agar supplemented with 100 µg/mL (Sigma, USA) grown at 37℃ and used for transformation. The genomic DNA of B. abortus 544 strain was extracted using a Bacterial Genomic DNA Purification Kit (ELPIS-Biotech, Korea), and the quality and purity of the extracted DNA was assessed using agarose gel electrophoresis. The target mdh gene (963 bp) was amplified using the following primer pairs: 5' AAT TC GGA TCC A TGG CAC GCA ACA AGA TT 3' (BamHI site underlined) and 5' AGG C GTC GAC TTA TTT CAG CGA CGG AGC 3' (SalI site underlined) designed based on the mdh gene sequence of B. abortus biovar strain 9-941 (accession No. YP_222574.1). The PCR mixture included 4 µL of bacterial genomic DNA, 25 µL of 2× PCR Master Mix Solution (iNtRON Biotechnology, Korea) and 10 picomoles of forward and reverse primers diluted to a final volume of 50 µL. The PCR amplification was optimized and the following parameters were used: heating at 95℃ for 5 min, followed by 30 cycles of denaturation at 95℃ for 1 min, annealing at 63℃ for 1 min and extension at 72℃ for 1 min, then final elongation at 72℃ for 5 min, after which the samples were held at 8℃. Analyses were conducted in a MyGenie96 Thermal Block thermal cycler (Bioneer, Korea). The PCR products were resolved using 1.5% Tris-acetate-EDTA (TAE) in an electrophoresis chamber containing 0.5× TAE buffer at 100 V for 25 min or until the dye indicator reached the target lane, stained with ethidium bromide and viewed under an ultraviolet transilluminator machine.
The amplified DNA product was purified using a Total Fragment DNA Purification Kit (iNtRON Biotechnology), digested with appropriate restriction enzymes (BamHI and SalI; Takara Bio, Japan), cloned into a pMAL vector (New England Biolabs, USA), transformed into E. coli DH5α competent cells, dispersed onto LB agar plates, and incubated at 37℃ for 16 to 18 h. The bacteria expressing the fusion plasmid were cultured overnight and transferred into ampicillin-containing LB broth. PCR amplifications were performed using primers specific for the mdh gene and colonies containing the recombinant plasmid were selected. For confirmation, the recombinant plasmid was extracted using a Plasmid Mini-Prep Kit (ELPIS-Biotech, Korea) and was sequenced from the vector-specified pMalE (Macrogen, Korea). The DNA sequence database was searched using BLASTN search algorithms (National Center for Biotechnology Information, USA).
The recombinant protein was induced through the modified procedure by adding isopropyl β-D-1-thiogalactopyranoside (IPTG) at a final concentration of 0.3 mM and further incubated at 37℃ for 4 h [3]. And then, the cells were then harvested by centrifugation at 3,000 × g for 10 min, resuspended in column buffer (20 mM Tris HCl, 200 mM NaCl, 1 mM EDTA, 0.1% Triton X100, 10% glycerol, pH 7.4), and frozen at -70℃ and thawed at least three times at 4℃. The suspensions were sonicated (BANDELIN electronic, Germany) at 10,000 Hz on ice, after which the supernatant was collected by centrifugation at 12,000 × g and 4℃ for 20 min. The supernatant was loaded onto a maltose resin column (Bio-Rad Laboratories, USA), after which the protein was purified according to the manufacturer's instructions and stored at -70℃ until use. An empty pMAL vector was utilized as a negative control and similar procedures were performed for insertion, induction and purification as that of rMDH. The purified recombinant protein was boiled for 5 min and diluted with an equal volume of 2× Laemmli sample buffer (2% sodium dodecyl sulfate [SDS], 10% glycerol, 2% β-mercaptoethanol, 0.004% bromophenol blue and 50 mM Tris HCl, pH 6.8). SDS-polyacrylamide gel electrophoresis (PAGE) was performed according to Dubray and Bézard [5], after which the samples were transferred onto Immobilon-P membranes (EMD Millipore, USA) using 1× transfer buffer (25 mM Tris, 192 mM glycine and 20% methanol) with a constant current of 2 mA/cm2 for 1 h in a semi-dry electroblot assembly (Bio-Rad Laboratories). The membrane was blocked with 5% skim milk (Becton and Dickinson Company) in 1× Tris-buffered saline-Tween 20 (TBS-T) for 30 min at room temperature with shaking, then incubated with Brucella-positive bovine antibody (1:1000) at the early (21 days post infection [PI]), middle (35 days PI) or late (70 days PI) stage of infection [8], and Brucella-positive mouse antibody (1:1000) at the early (10 days PI), middle (30 days PI) or late (60 days PI) stage of infection at 4℃ overnight. As a control, proteins were also incubated using Brucella-negative bovine and mouse sera (1:1000). Brucella-positive sera from our previous studies, which were collected from experimentally infected animals and assessed using the buffered plate agglutination test and Rose Bengal test according to the OIE standard procedures of serological tests for brucellosis, were utilized [78]. The membrane was washed with 1× TBS-T and incubated with horseradish peroxidase-conjugated protein G (1:1000 dilution; Thermo Scientific, USA) for 1 h. The immunogenicity of the recombinant protein was then detected using a luminal-coumaric acid-H2O2 detection solution (ATTO Corporation, Japan) and exposed in a Molecular Imager ChemiDoc XRS+ system machine (Bio-Rad Laboratories). Western blotting was performed using at least two representative sera from each stage of infection.
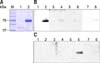
A specific band corresponding to the B. abortus mdh gene was amplified from genomic DNA of B. abortus 544 strain and analyzed as a single band with the correct band size (963 bp) on 1.5% (w/v) agarose gel (Fig. 1). The PCR product was cloned into pMAL expression vector and successfully transformed into competent E. coli DH5α cells as confirmed by PCR using the mdh gene primers and sequencing (homology, 99%). In addition, rMDH protein in pMAL vector was successfully induced using IPTG, then purified using maltose resin column with a molecular size of approximately 76 kDa (Fig. 2) on 10% SDS-PAGE. The immunoreactivity of the cloned protein was evaluated by Western blot assay and a stronger reaction was observed with Brucella-positive bovine serum in the early stage (21 days PI) of infection and with Brucella-positive mouse serum in the late stage (60 days PI) of infection (panels B and C in Fig. 3). The rMDH did not react with either Brucella-negative bovine or mouse serum (panels B and C in Fig. 3).
Brucellosis is a widespread infectious disease that persists in domestic livestock animals and remains a significant source of human infection worldwide [3]. Extensive efforts have been made to prevent the disease in animals through vaccination programs; however, current available vaccines have several drawbacks, including interference with diagnosis, antibiotic resistance and residual virulence [12]. Clinical diagnosis of the disease is also difficult to establish because of diverse factors such as variable time of incubation, non-specific clinical signs and occurrence of false-positive reactions [3]. Consequently, the present study cloned the mdh gene of Brucella and investigated its immunogenicity using Brucella-positive and -negative sera for future evaluation of its efficacy as a subunit vaccine candidate for comparison with other reported studies, as well as for serological test development to determine its specificity as a potential diagnostic tool.
In our previous study, malate dehydrogenase (MDH) was one of the immunogenic Brucella proteins reactive in the middle infection period (30 days) with the exclusion of cross-reaction with Y. enterocolitica O:9 and non-infected mouse sera [7]. This enzyme has been related to B. abortus pathogenesis, which may act as a new virulent factor, and MDH activity can be inhibited using copper, zinc or lead to block the TCA metabolism pathway, which provides new insights to control B. abortus infection [14]. Previous studies have shown that B. abortus MDH is an antigenic protein that plays an important role during infection of cervid and bovine hosts [9]. Moreover, mice vaccinated with rMDH protein displayed the most significant reduction in bacterial co-localization among several recombinant proteins tested, suggesting that B. abortus MDH enhanced protection against Brucella infection [10].
In this study, B. abortus mdh gene expression, induction and purification of the recombinant protein were successfully performed, and the purified recombinant protein was approximately 76 kDa. This protein was further evaluated for immunogenicity with Brucella-infected bovine and mouse sera at different stages of infection.
In summary, the purified rMDH protein from B. abortus expressed in pMAL vector was reactive to Brucella-positive bovine serum at an early stage (21 days PI) of infection, but not with Brucella-negative bovine serum, suggesting its applicability as a specific antigenic component in development of serological diagnostic tools for the early detection of bovine brucellosis. In addition, it was reactive to Brucella-positive mouse serum at the late stage (60 days PI) of infection, but not to Brucella-negative mouse serum, suggesting its potential as a subunit vaccine candidate using the cattle or mouse model. However, the efficacy of this recombinant protein needs further evaluation.
Figures and Tables
Fig. 1
Amplification of Brucella (B.) abortus mdh gene. PCR products on 1.5% (w/v) agarose gel. Lane 1, DNA marker; Lane 2, single expected band of mdh gene (963 bp, arrow).
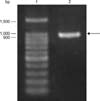
Fig. 2
Expression and purification of B. abortus malate dehydrogenase (MDH) in pMAL expression system vector with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Coomassie brilliant blue staining. Lane 1, Protein Marker; Lane 2, total E. coli DH5α lysates; Lane 3, uninduced rMDH; Lane 4, induced unpurified rMDH; Lane 5, purified rMDH (approximately 76 kDa, arrow).

Fig. 3
Expression, purification and immunogenicity of B. abortus MDH in pMAL expression system vector. (A) SDS-PAGE profiles of rMDH (approximately 76 kDa) and empty pMAL vector (approximately 43 kDa) separated and stained with Coomassie brilliant blue. Lane M, protein marker; Lane 1, empty pMAL vector; Lane 2, purified rMDH. (B) Western blot analysis using Brucella-positive bovine serum at early (Lane 1, empty pMAL vector; Lane 2, purified rMDH), middle (Lane 3, empty pMAL vector; Lane 4, purified rMDH) and late stages of infection (Lane 5, empty pMAL vector; Lane 6, purified rMDH), and Brucella-negative bovine serum (Lane 7, empty pMAL vector; Lane 8, purified rMDH). (C) Western blot analysis using Brucella-positive mouse serum at early (Lane 1, empty pMAL vector; Lane 2, purified rMDH), middle (Lane 3, empty pMAL vector; Lane 4, purified rMDH) and late stages of infection (Lane 5, empty pMAL vector; Lane 6, purified rMDH), and Brucella-negative mouse serum (Lane 7, empty pMAL vector; Lane 8, purified rMDH).
Acknowledgments
The work was supported the Strategic Initiative for Microbiomes in Agriculture and Food, Ministry of Agriculture, Food and Rural Affairs, Korea.
References
1. Al Dahouk S, Tomaso H, Nöckler K, Neubauer H, Frangoulidis D. Laboratory-based diagnosis of brucellosis– a review of the literature. Part II: serological tests for brucellosis. Clin Lab. 2003; 49:577–589.
2. Carvalho Neta AV, Mol JPS, Xavier MN, Paixão TA, Lage AP, Santos RL. Pathogenesis of bovine brucellosis. Vet J. 2010; 184:146–155.

3. Cassataro J, Pasquevich K, Bruno L, Wallach JC, Fossati CA, Baldi PC. Antibody reactivity to Omp31 from Brucella melitensis in human and animal infections by smooth and rough brucellae. Clin Diagn Lab Immunol. 2004; 11:111–114.

4. Clavijo E, Díaz R, Anguita Á, García A, Pinedo A, Smits HL. Comparison of a dipstick assay for detection of Brucella-specific immunoglobulin M antibodies with other tests for serodiagnosis of human brucellosis. Clin Diagn Lab Immunol. 2003; 10:612–615.

5. Han X, Tong Y, Tian M, Zhang Y, Sun X, Wang S, Qiu X, Ding C, Yu S. Enzymatic activity analysis and catalytic essential residues identification of Brucella abortus malate dehydrogenase. ScientificWorldJournal. 2014; 2014:973751.
6. Ko J, Splitter GA. Molecular host-pathogen interaction in brucellosis: current understanding and future approaches to vaccine development for mice and humans. Clin Microbiol Rev. 2003; 16:65–78.

7. Lee JJ, Simborio HL, Reyes AWB, Kim DG, Hop HT, Min W, Her M, Jung SC, Yoo HS, Kim S. Proteomic analyses of the time course responses of mice infected with Brucella abortus 544 reveal immunogenic antigens. FEMS Microbiol Lett. 2014; 357:164–174.
8. Lee JJ, Simborio HL, Reyes AWB, Kim DG, Hop HT, Min W, Her M, Jung SC, Yoo HS, Kim S. Immunoproteomic identification of immunodominant antigens independent of the time of infection in Brucella abortus 2308-challenged cattle. Vet Res. 2015; 46:17.
9. Lowry JE, Goodridge L, Vernati G, Fluegel AM, Edwards WH, Andrews GP. Identification of Brucella abortus genes in elk (Cervus elaphus) using in vivo-induced antigen technology (IVIAT) reveals novel markers of infection. Vet Microbiol. 2010; 142:367–372.

10. Lowry JE, Isaak DD, Leonhardt JA, Vernati G, Pate JC, Andrews GP. Vaccination with Brucella abortus recombinant in vivo-induced antigens reduces bacterial load and promotes clearance in a mouse model for infection. PLoS One. 2011; 6:e17425.
11. McGiven JA, Stack JA, Perrett LL, Tucker JD, Brew SD, Stubberfield E, MacMillan AP. Harmonisation of European tests for serological diagnosis of Brucella infection in bovines. Rev Sci Tech. 2006; 25:1039–1053.
12. Olsen SC. Recent developments in livestock and wildlife brucellosis vaccination. Rev Sci Tech. 2013; 32:207–217.

13. Pajuaba AC, Silva DAO, Almeida KC, Cunha-Junior JP, Pirovani CP, Camillo LR, Mineo JR. Immunoproteomics of Brucella abortus reveals differential antibody profiles between S19-vaccinated and naturally infected cattle. Proteomics. 2012; 12:820–831.

14. Park SJ, Cotter PA, Gunsalus RP. Regulation of malate dehydrogenase (mdh) gene expression in Escherichia coli in response to oxygen, carbon, and heme availability. J Bacteriol. 1995; 177:6652–6656.

15. Seleem MN, Boyle SM, Sriranganathan N. Brucellosis: a re-emerging zoonosis. Vet Microbiol. 2010; 140:392–398.

16. Trant CG, Lacerda TLS, Carvalho NB, Azevedo V, Rosinha GMS, Salcedo SP, Gorvel JP, Oliveira SC. The Brucella abortus phosphoglycerate kinase mutant is highly attenuated and induces protection superior to that of vaccine strain 19 in immunocompromised and immunocompetent mice. Infect Immun. 2010; 78:2283–2291.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download