Abstract
To assess the effects of a single supraphysiological postnatal administration of a progestogen on uterine glands in dogs, 10 females were randomly assigned to a medroxyprogesterone acetate 35 mg (MPA; n = 6) or placebo (n = 4) group within the first 24 h of birth. The safety of the treatment was also evaluated. A transient mild clitoris enlargement appeared in MPA-treated females. Microscopic postpubertal uterine assessment revealed the presence of uterine glands in all cases without significant differences in the area occupied by the glands per µm2 of endometrium nor in the height of the uterine epithelium.
In both precocial and altricial mammals such as mice, rats, sheep and pigs, uterine adenogenesis begins during the first 2 weeks of postnatal life [15]. Specifically, canine adenogenesis is initiated by the end of postnatal week 1 and both epithelial and stromal cell proliferation patterns decrease systematically over time, such that endometrial gland development is essentially completed by week 6 to 8 [2].
In previous studies carried out in sheep [9] and mice [3], neonatal administration of progestins permanently inhibited the genesis of uterine glands causing adult infertility. Although abundant international reports [36] support investigation of the potential use of neonatal progestins in small animal permanent contraception, a preliminary report showed that a progestogen (medroxyprogesterone acetate 10 mg/kg every 3 weeks) did not inhibit uterine adenogenesis when serially administered up to the age of 6 months [10]. In this respect, it has been shown that there is a limited time window of susceptibility to progestin-mediated inhibition of gland development and that administration outside this period leads to a failure of complete or permanent abolishment of adenogenesis [3]. Furthermore, a protocol including eight progestin injections would be impractical and unsafe [8].
Based on previous reports, it was hypothesized that a single supraphysiological progestin administered before the onset of uterine adenogenesis permanently abolishes gland development in domestic canids. Thus, the goal of the present study was to assess the effects of a single postnatal administration of medroxyprogesterone acetate on postpubertal uterine glands in dogs. The clinical safety of this treatment was also evaluated.
Ten cross-bred, female littermates born in our institutional colony were sexed and identified at birth. The study was reviewed and approved by the Animal Care and Use Committee of the Veterinary School of the National University of La Plata and all experiments were conducted under the guidelines established in The Guide for The Care and Use of Laboratory Animals, USA.
Puppies (333.2 ± 17.3 g body weight) were randomly assigned to one of the following treatment groups within the first 24 h of birth: medroxyprogesterone acetate (MPA) 35 mg subcutaneously (s.c.; n = 6; Singestar; Laboratories Konig, Argentina) or placebo (PLC) : 0.6 mL corn oil s.c. (n = 4).
All animals were followed up until the first pubertal signs appeared. During the follow up period, they were observed for sexual behavior, physically examined and weighed. Eventual appearance of clinical side effects was also recoded. Vaginal cytology was carried out three times per week from the fourth month of age onwards. Puberty was defined by finding more than 80% superficial keratinized cells and a clean background in the vaginal smears accompanied by typical estrous behavior.
As the female dogs attained puberty, peripheral blood samples were taken 14 days after estrus for ovulation assessment (progesterone > 5 ng/mL; Elecsys; Roche Diagnostics, Germany), after which all the bitches underwent ovariohysterectomy.
After surgical removal, the uteri were macroscopically examined, weighed, and internally inspected. Cross-sections of each uterine horn were fixed and processed for histological evaluation. The uteri were examined for the presence or absence of endometrial glands. The area occupied by uterine glands per µm2 of endometrium over the total area of each microscope field was measured by planimetry. The height of the uterine epithelium was assessed by counting 100 cells in a total of 10 images per uterus taken with a 20× objective.
Differences between groups were carried out by Fisher's Exact and Student's t tests. Data were expressed as mean ± SEM and p values < 0.05 were considered significant.
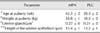
Age and body weight at puberty did not differ between groups (Table 1). The MPA-treated bitches presented mild clitoris enlargement from 2 to 17 postnatal weeks, after which they gradually normalized. All pubertal females showed normal sexual behavior, ovulated during heat and presented no side effects.
Uterine wet weight was not different between groups and its gross internal evaluation revealed no abnormalities. Microscopic assessment of the uteri showed the presence of uterine glands in all the cases. In the MPA-treated dogs, the area occupied by uterine glands per µm2 of endometrium and the height of the uterine epithelium did not differ from the PLC group (Table 1, panels A and B in Fig. 1).
This is the first study that describes the effect of a single postnatal supraphysiological dose of a potent and time-released progestin, medroxyprogesterone acetate, on canine uterine adenogenesis. This progestin treatment did not seem to affect somatic development or age at puberty. The transient clitoris abnormalities observed in MPA-treated females could be due to the androgenic effect of progestins [7]. Converse to what has been reported for the neonatal administration of other steroids in rodents [11], in this study, progestin did not modify sexual behavior. The hypothalamic-pituitary-ovarian axis functionality was apparently not affected as ovulation occurred in all the animals.
Histological assessment examination revealed that the single supraphysiologic dose of the long term release progestin did not prevent the genesis of uterine glands in these bitches. It is difficult to interpret these findings in contraposition to what has been extensively described for ewes and mice [36]. Interestingly, similar treatments have also failed to block uterine adenogenesis in porcine species [4]. In these dogs neither the area occupied by uterine glands nor the height of the uterine epithelium was reduced by the progestin. Furthermore, epithelium height had a tendency to be higher in the MPA group.
Although, this pharmacological protocol appeared to be clinically safe, there is still the potential for eventual long term side effects. Nevertheless, a single postnatal supraphysiological dose of medroxyprogesterone acetate did not ablate uterine adenogenesis or cause short or medium term side effects in domestic dogs.
Figures and Tables
 | Fig. 1Uterus of female dogs treated postnatally with medroxyprogesterone acetate 10 mg/100 g subcutaneously (s.c) (A) or Placebo s.c. (B) and hysterectomized after pubertal estrous cycle. Notice normal uterine gland pattern in both treatments. *Stroma. †Glandular epithelium. H&E stain. Scale bars = 20 µm. |
Acknowledgments
This study was partially funded by the National Agency of Scientific Promotion and Technology (PICT 426-2014) and CONICET (PIP 0095). TP, MF and MLP are Research Fellows and CB and CG are Career Scientists of CONICET, Argentina. The authors thank Mr. Ruben Mario for histological technical work.
References
1. Allison Gray C, Bartol FF, Taylor KM, Wiley AA, Ramsey WS, Ott TL, Bazer FW, Spencer TE. Ovine uterine gland knock-out model: effects of gland ablation on the estrous cycle. Biol Reprod. 2000; 62:448–456.

2. Cooke PS, Borsdorf DC, Ekman GC, Doty KF, Clark SG, Dziuk PJ, Bartol FF. Uterine gland development begins postnatally and is accompanied by estrogen and progesterone receptor expression in the dog. Theriogenology. 2012; 78:1787–1795.

3. Cooke PS, Ekman GC, Kaur J, Davila J, Bagchi IC, Clark SG, Dziuk PJ, Hayashi K, Bartol FF. Brief exposure to progesterone during a critical neonatal window prevents uterine gland formation in mice. Biol Reprod. 2012; 86:63.
4. Cooke PS, Spencer TE, Bartol FF, Hayashi K. Uterine glands: development, function and experimental model systems. Mol Hum Reprod. 2013; 19:547–558.

5. Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW, Spencer TE. Developmental biology of uterine glands. Biol Reprod. 2001; 65:1311–1323.
6. Gray CA, Taylor KM, Bazer FW, Spencer TE. Mechanisms regulating norgestomet inhibition of endometrial gland morphogenesis in the neonatal ovine uterus. Mol Reprod Dev. 2000; 57:67–78.

7. Romagnoli S, Concannon PW. Clinical use of progestins in bitches and queens: a review. In : Concannon PW, England GCW, Verstegen J, Linde-Forsberg C, editors. Recent Advances in Small Animal Reproduction. New York: International Veterinary Information Service;2003.
8. Selman PJ, Mol JA, Rutteman GR, Rijnberk A. Progestins and growth hormone excess in the dog. Acta Endocrinol (Copenh). 1991; 125:Suppl 1. 42–47.
9. Spencer TE, Gray CA. Sheep uterine gland knockout (UGKO) model. Methods Mol Med. 2006; 121:85–94.

10. Teixeira NS, Martins BB, Volpato R, Freitas PMC, Lopes MD, Laufer-Amorim R, Luz MR. Post natal progestogen administration does not suppress endometrial glands development in the bitch. Abstracts of Seventh International Symposium on Canine and Feline Reproduction. In : 15th Congress of the European Veterinary Society for Small Animal Reproduction; 26-29 July 2012; Whistler, Canada.
11. Thomas DA, Howard SB, Barfield RJ. Influence of androgen on the development of sexual behavior in the rat II Time and dosage of androgen administration during the neonatal period and masculine and feminine copulatory behavior in females. Horm Behav. 1983; 17:308–315.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download