Abstract
Our objective was to evaluate the effectiveness of skin-stretching devices for closing defects on the extremities of dogs. Antebrachial skin defects were created on the limbs of 24 dogs randomly divided into three groups. Skin stretchers included staples and sutures passing through them (group A), sutures and hypodermic needles (group B), and Pavletic device (group C). Wounds on the left were further undermined in all groups. Tension and blood perfusion were assessed. After removing the stretchers on day 3, the defects were sutured and wound healing was clinically scored. Histological variables evaluated were cellular infiltration, edema, collagen orientation, and thickness of epidermis. Significant differences in tension were found among groups (p < 0.0005) and between measurement times for undermined (p = 0.001) or non-undermined (p < 0.0005) wounds. In contrast, blood perfusion values did not differ significantly. Clinical scores for group B seemed to be better than those for groups A and C, but differences were not significant. Primary wound closure using the Pavletic device was not feasible. No significant differences in histological variables were found between groups. Skin stretching with staples or hypodermic needles resulted in successful wound management with minor side effects on skin histology and circulation.
Skin defects on the limbs of dogs are often difficult to close mainly due to the absence of skin abundance. Skin has distinctive viscoelastic properties such as creep and stress relaxation [3,12]. Skin-stretching devices harness these properties and facilitate delayed primary closure of wounds. Creep dictates that skin will extend as it is stretched at a constant tension [30]. Stress relaxation indicates that if skin is stretched a set distance, the amount of tension required to keep the skin stretched will slowly decrease over time [3,12,18]. Skin stretchers exert controlled mechanical load on large deficits that causes gradual traction to the skin. Thus, complete wound closure can be achieved within a short period of time.
Various devices have been proposed for promoting delayed primary wound closure. Hypodermic or spinal needles [1], Kirschner wires [5] and staples combined with sutures [9] or orthopedic wire, polyamide [6] or silastic Dacron strips [2], a suture tension adjustment reel device [8], a wise bands device [4], and Hirshowitz's device [15] have all been used in human clinical practice. Furthermore, Pavletic created a stretching device consisting of adherent skin pads applied to opposing sides of the defect and adjustable elastic straps that engage the skin pads [23,24]. This device has been used in dogs [23] and rabbits [7] to close defects on the neck and trunk, but its application on lower extremities has been considered almost ineffective.
Delayed, mechanically assisted, primary closure stretches local skin with the same color and texture of the surrounding tissues [4]. Another advantage that skin stretchers have over tissue expanders or flaps is that there is no need for a second surgery [21,23,25,26]. Most techniques used for skin stretching are simple and inexpensive. They can also be used for infected wounds, in most cases can be applied under local anesthesia, and are associated with decreased healing time and hospitalization [17]. To the best of our knowledge, the effects of various skin-stretching devices on the closure of wounds on the limbs of dogs, as well as changes in skin microcirculation and histology, have not been studied. We decided to evaluate the effectiveness of Pavletic device among other skin stretchers using staples and needles, although its application at this specific site may face difficulties due to paucity of space or skin elasticity. The purpose, therefore, of the present study was to evaluate the effectiveness of skin-stretching devices on the closure of skin defects on the extremities of dogs and to determine the effects of stretching on cutaneous microcirculation, histology, and overall healing.
The present study was approved by the ethics committee of the Hellenic State Veterinary Authorities on animal care and use. Twenty-four healthy purpose bred, adult mixed breed dogs 1~4 years old were used (10 castrated males and 14 spayed females). A complete physical examination, complete blood count, serum biochemical analyses, and urinalysis were performed on each dog before the study commenced. The animals were housed in indoor individual runs and had outdoor access twice daily. Commercial dry maintenance diets were given twice daily and water was available ad libitum.
Following the creation of skin defects on both forearms, the animals were randomly divided in three groups of eight dogs each. In group A, skin staples were used for skin stretching. In group B, the needle of an intravenous catheter was inserted along each side of the wound. In group C, a Pavletic skin-stretching device was applied.
The dogs were premedicated with acepromazine (0.05 mg/kg, im, Acepromazine; Alfasan International, The Netherlands) and butorphanol (0.1 mg/kg, iv, Butadors; Richter Pharma, Austria). Anesthesia was induced with 2.5% sodium thiopental (8 mg/kg, iv, Pentothal; Abbott Laboratories, USA) and maintained with isoflurane (1~2%, Isoflo; Abbott Laboratories) in oxygen (1.5 L/min). Preoperatively, cefuroxime (20 mg/kg, iv, Zinacef; GlaxoSmithKline, UK) and carprofen (2 mg/kg, iv, Rimadyl; Pfizer, USA) were administered. Both forelimbs were clipped from the middle of the humerus to the carpus and prepared for aseptic surgery.
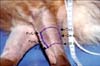
On day 1, one skin defect was created by a No. 10 blade on the craniolateral surface of each mid-forearm (Fig. 1). The defect had a length of 5 cm and a width ranging from 3.5~7 cm in order to be equal to 50% of the circumference of the mid-forearm. Undermining of the wound edges at a distance of 2 cm in left extremity was performed. For group A (Fig. 2), skin staples (Proximate plus MD; Ethicon, USA) in groups of two or three were placed circumferentially and perpendicular to the skin edges [9]. The groups of staples were placed 1 cm apart from each other and 0.5 cm away from the wound edge. Simple interrupted polypropylene 0 sutures (Prolene; Ethicon) were passed through the staples on each opposing side of the wound and tied over a cylinder (made from polypropylene thoracic tube cases by cutting them into 10 cm sections and by creating side holes) after passing through the side holes. The cylinder was placed over the wound on top of a sterile non-adherent, semi-occlusive pad (Melolin; Smith & Nephew, UK). For group B (Fig. 3), the needle of an intravenous catheter (catalogue no. 16; Abbott, Ireland) was inserted along each wound side approximately 0.5 cm from the wound edge [1,25]. Simple interrupted polypropylene 0 sutures were passed around the needles on each opposing side of the wound and tied over a cylinder as in group A. For group C (Fig. 4), a Pavletic skin-stretching device (X Banders; M.M. Pavletic, USA) was applied [23,24]. Specifically, three adherent skin pads of the device, measuring 0.5 × 5 cm, were placed next to each other on opposite sides of the defect and attached to the skin by using the cyanoacrylate glue and simple interrupted polyamide 3/0 sutures. The adjustable elastic straps engaged the pads of one side of the defect to the pads of the opposing side.
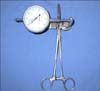
During suture tightening, tension was measured with a special device that consisted of a dynamometer attached to haemostatic forceps (Fig. 5) provided by the Centre for Research and Technology of Greece. Care was taken to not exceed a limit of 3.5 kg [15]. Tips of the forceps were passed through the suture loops on opposite wound sides and the force required to pull each side of the defect to the midline was measured [19]. Thereafter, the wounds were bandaged with sterile non-adherent, semi-occlusive pads (Melolin; Smith & Nephew) and Elizabethan collars were placed on the dogs. Under general anesthesia, dogs in groups A and B underwent three cycles of intermittent skin stretching (cycle loading) 24 h after creation of the defects (day 2). Forces of 1.5~2 kg were applied for 2~3 min followed by 1-min periods of relaxation [25]. The sutures were tightened once again and tension was measured. On day 3, the skin stretchers were removed with the canines under general anesthesia. For group C, cycle loading was not performed. The elastic strapsattached to the pads of one wound side were stretched prior to engaging the pads of the opposing side. The straps were adjusted every 24 h for a total of 48 h.
On day 3 (48 h after creation of the defects) following removal of the stretchers, primary closure of the skin wounds was performed for groups A and B (Fig. 6) but not group C. Closure was achieved in a two-layer fashion using polyglecaprone 3/0 (Monocryl; Ethicon) in a continuous pattern and polypropylene 2/0 in a simple interrupted pattern. All wounds were dressed using the bandage pads mentioned above. The bandages were changed daily until suture removal on day 15 (groups A and B) or wound healing (group C). Postoperatively, all animals received cefuroxime (20 mg/kg, im, bid, Zinacef; GlaxoSmithKline) for at least 10 days and carprofen (2 mg/kg, per os, bid, Rimadyl; Pfizer) for 5 days.
Following primary wound closure in groups A and B, and wound healing in group C, healing was clinically scored daily until suture removal (day 15). The following modified scale was used: 1 = no visible reaction; 2 = minimal swelling or erythema; 3 = suture line inflammatory reaction at least 1-cm thick with pain or redness; 4 = seroma or abscess formation, and 5 = dehiscence, skin necrosis, or impossible primary closure [10]. Scoring was performed by a clinician (M. Karayannopoulpou) who was not aware of which stretching devices had been applied in each group.
Cutaneous microcirculation was assessed by laser-Doppler flowmetry (LDF). The animals were acclimatized to the study room (20°~22℃) at least 30 min before anesthesia [26]. LDF measurements were performed under general anesthesia using the same protocol as for wound creation, with the dogs' body temperature ranging between 38~39℃. A laser-Doppler velocimeter (Periflux 4001 Master; Perimed, Sweden) with a standard right angle probe was used. Blood perfusion was assessed (Fig. 1) at six predetermined sites (three on the frontal and three on the caudal side of the defect at the upper, middle, and lower part and at a distance of 0.5 cm from the skin stretchers). LDF was performed just before creation of the defect (day 1), 24 h after defect creation, prior to cycle loading and suture tightening (day 2), and 48 h after wound creation (day 3). Each time, five separate readings were recorded at 5-min intervals to obtain a mean value for proper interpretation [26]. Values are expressed in perfusion units (PU).
Samples for histological evaluation were taken on the day of wound creation (from the excised skin; control specimen), on day 3 (following primary wound closure), and at the time of suture removal (day 15). Specimens were obtained using a 6-mm biopsy punch (Biopsy punch; Jørgen Kruuse, Denmark) while the dogs were under general anesthesia as previously described, at a distance of 0.5 cm from the wound margin or suture line (day 3 and 15). The specimens were pinned to a flat cork surface and fixed in 10% neutral-buffered formalin. They were labeled so that the pathologists (D. Psalla, N. Papaioannou) were not aware of the animal number, biopsy day or group corresponding to the specimen. After routine processing (dehydration in a series of alcohols, clearing in xylene and paraffin embedding), the samples were cut into sections 4- to 6-µm thick and stained with Hematoxylin-Eosin (H&E; Merk, Germany) along with Van Gieson stain (Merk) for elastin.
The stained sections were microscopically examined. The degree of cellular infiltration (neutrophils, eosinophils, macrophages, lymphocytes, and plasma and mast cells), edema, collagen orientation, and thickness of the epidermis were evaluated using the following criteria for scoring. For cellular infiltration, sparsely scattered inflammatory cells arranged in a random fashion in the dermis were considered normal (score 0). Detection of three to 10, 11 to 30, or ≥ 31 cells per high power field (400 ×) in the wound tissue was considered mild (score 1), moderate (score 2), or substantial (score 3) infiltration, respectively [13]. Absence of cells separating from collagen was considered a lack of edema (0), slight separation was considered mild edema (1), a separation of 30 to 50 µm was considered moderate edema (2), and a separation of > 50 µm was considered substantial edema (3) [13]. Orientation of collagen bundles (the number of bundles that ran parallel to the skin surface) was graded according to the following semi-quantitative scoring system [28]: orientation exclusively perpendicular to the skin surface (0), orientation predominantly perpendicular to the skin surface (1), random orientation (2), orientation predominantly parallel to the skin surface (3), and orientation parallel to the skin surface (4). Epidermal thickness was evaluated relative to that of normal epidermis of the excised skin on day 0 (control specimen) and assigned a score of 0 (thickness similar to that of normal epidermis), 1 (slightly decreased thickness), 2 (moderately decreased thickness), and 3 (substantially decreased thickness).
Data are expressed as the mean ± standard deviation (SD). Normality of the data distribution was assessed with a Shapiro-Wilk test. To assess any statistically significant differences, variables were compared among all groups as well as between undermined and non-undermined wounds at each time point or between measurement times in each group. A general linear model for repeated measures (for tension and LDF) or the Wilcoxon paired-sample ranks test (for histological parameters) was used to make these comparisons. P values ≤ 0.05 were considered significant. All statistical analyses were performed using SPSS software (ver. 15.0; SPSS, USA).
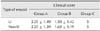
Mean values for tension in all groups measured on days 1 and 2 are presented in Table 1. Irrespective of measurement time, statistically significant differences in tension values (p < 0.0005) were found among all groups. The lowest and highest mean values were recorded for groups B and C, respectively. Irrespective of group, significantly decreased tension was observed on day 2 compared to day 1 in both undermined (p = 0.001) and non-undermined (p < 0.0005) wounds.
The mean clinical scores for the undermined (left limb) and non-undermined (right limb) wounds during the healing period (from wound closure on day 3 to suture removal on day 15) in groups A and B as well as group C (although primary wound closure was impossible) are presented in Table 2. Wound healing seemed to be better, although not significantly, in group B compared to group A. Overall, no significant differences in clinical scores were observed between the undermined and non-undermined wounds.
Mean blood perfusion values at the six different sites during the examined period in undermined and non-undermined wounds as well as the three groups are presented in Table 3. No statistically significant differences in mean LDF values were found among groups A, B, and C. On day 2, the mean LDF value decreased but not significantly compared to day 1 irrespective of group. On day 3, this value significantly increased compared those recorded on day 1 (p = 0.048) and day 2 (p = 0.012). No significant differences in blood perfusion were found between undermined and non-undermined wounds in any group. Some significant differences in blood perfusion were recorded between the sites of LDF measurements. Irrespective of location in the wound (upper, middle, or lower portion) or undermining of the wound (left or right limb), blood perfusion measurements taken on day 3 differed significantly between the frontal and caudal wound side in groups A (p < 0.0005) and C (p = 0.048).
Mean values of the examined histological variables for the three groups are presented in Table 4. A mild to moderate increase in cellular infiltration was found in all wounds. Additionally, moderate to substantial edema was evident on day 3 in all the groups. On day 15, edema was mild to moderate. Collagen orientation was mainly parallel to the skin surface. Epidermal thickness was decreased on day 3 as well as day 15 to a lesser extent. No statistically significant differences were observed for any of the histological variables.
Wound closure on the extremities of dogs poses a challenge to veterinary surgeons due to the paucity of elastic skin. The most appropriate method depends on wound dimensions, exposed underlying structures, presence of infection, technical demands, operative skills, and treatment costs. Presuturing, skin undermining, local and axial flaps, pouch flaps, grafts, skin expanders, stretching devices, microvascular free tissue transfer, and secondary healing could be used for closing defects on the limbs [11,24,27]. Skin stretchers that were effective for groups A and B in our study had been evaluated in a limited number of clinical cases in humans [1,9,25], but had never been previously used in dogs. On the other hand, we evaluated the ability of the Pavletic device to manage wounds on the extremities for the first time. In the present study, better wound healing results (clinical scoring) observed in group B could be due to the fact that tension across the wound margins was more evenly distributed by using hypodermic needles compared to staples, which had a smaller contact area. On the contrary, the Pavletic stretching device used in group C did not effectively promote wound closure on the extremities. Apart from the absence of skin abundance, one possible reason for this result might be the fact that the pads were placed near the wound edges. Pavletic recommends that they should be placed approximately 5~10 cm away from the wound margins [23]; however, this was not feasible for wounds on canine extremities.
During skin stretching, a safe threshold of the applied force ranges from 0.5 to 4 kg [15]. Greater forces can stretch collagen fibers and small vessels, resulting in the reduction or cessation of blood perfusion and subsequent necrosis of skin margins [1,30]. The tension applied in the present study ranged from 1.5 to 3 kg and was considered safe. When used in combination with cycle loading, this technique contributed to skin elongation as described in other studies [8,18]. Significantly decreased tension found on day 2 compared to day 1 in our study was probably due to stress relaxation [3]. The absence of any statistically significant differences in tension between undermined and non-undermined wounds indicated that the combination of undermining and stretching does not cause further tension reduction. This is in agreement with results from other studies showing that skin-stretching devices reduce tension for skin closure more effectively than undermining [16,21]. Mackay et al. [19], however, reported that undermining was more important than intra-operative tissue expansion.
Skin perfusion has been evaluated using various methods. LDF is a noninvasive, simple, and accurate method for measuring cutaneous microcirculation [20]. In the present study, no significant decreases in blood flow were observed between days 1 and 2. This is probably because measurements were taken 24 h after suture tightening when stress relaxation had already occurred. Hallock and Rice [14] reported a decrease in blood flow immediately after rapid tissue expansion, but there have been no reports about perfusion changes during skin stretching. Rapid skin expansion in rats results in a significantly sustained increase in mitotic activity due to mechanical stress and tissue cell hypoxia that stimulates angiogenesis [17]. Moreover, Alex et al. [2] reported that stretchers produced greater angiogenesis 1 week after application compared to expanders in a porcine model. Similarly, in our study significantly increased blood perfusion observed on day 3 compared to days 1 and 2 may indicate increased angiogenesis. The significant difference in blood perfusion found between the frontal and caudal wound side may be attributed to the relevant proximity to the cephalic vein. Furthermore, the fact that wound undermining did not significantly affect blood perfusion could be due to preservation of the subdermal plexus blood supply [9].
Histological changes produced by skin-stretching devices have not been previously studied in canines. Increased epidermal thickness and decreased dermal thickness has been reported after skin stretching in humans [22] whereas increased thickness of both the epidermis and dermis was observed in pigs [2,29]. In our study, increased dermal thickness (increased edema) and thinning of the epidermis were probably due to the two-dimensional nature of the applied forces [2].
In conclusion, skin stretching using staples and hypodermic needles was shown effective for closing large defects on the extremities of dogs in contrast to the Pavletic device. Tension applied to the skin, ranging from 1.5 to 3 kg, was found to be safe. Skin stretching had minor side effects on skin histology and circulation. On the other hand, skin undermining did not manage to further reduce the tension for wound closure.
Figures and Tables
 | Fig. 1Markings for the skin defect on the craniolateral surface of the mid-forearm. Note the sites for measuring blood perfusion using LDF. F; frontal wound side, C; caudal wound side, u; upper wound, m; middle wound, l; lower wound, L; left limb (undermined wounds). |
Table 1
Mean values and standard deviation (mean ± SD) of tension (expressed in g) in undermined and non-undermined wounds in the three groups
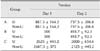
Table 2
Mean values (± SD) of clinical scores for undermined (left limb) and non-undermined (right limb) wounds in the three groups
Acknowledgments
The authors would like to express their sincere appreciation and thank Professors Angelos Dessiris and Lambis Lazaridis for their significant guidance in the present study. The investigation was supported by a grant (no. 3422) from the Hellenic State Scholarships foundation.
References
1. Abramson DL, Gibstein LA, Pribaz JJ. An inexpensive method of intraoperative skin stretching for closure of large cutaneous wounds. Ann Plast Surg. 1997; 38:540–542.

2. Alex JC, Bhattacharyya TK, Smyrniotis G, O'Grady K, Konior RJ, Toriumi DM. A histologic analysis of three-dimensional versus two-dimensional tissue expansion in the porcine model. Laryngoscope. 2001; 111:36–43.

3. Armstrong DG, Sorensen JC, Bushman TR. Exploiting the viscoelastic properties of pedal skin with the sure closure skin stretching device. J Foot Ankle Surg. 1995; 34:247–253.

4. Barnea Y, Gur E, Amir A, Leshem D, Zaretski A, Shafir R, Weiss J. Our experience with Wisebands: a new skin and soft-tissue stretch device. Plast Reconstr Surg. 2004; 113:862–869.

5. Bashir AH. Wound closure by skin traction: an application of tissue expansion. Br J Plast Surg. 1987; 40:582–587.

6. Blomqvist G, Steenfos H. A new partly external device for extension of skin before excision of skin defects. Scand J Plast Reconstr Surg Hand Surg. 1993; 27:179–182.

7. Bostrom B, Wilson H, Radlinsky M. The use of an external skin-stretching device for wound management in a rabbit (Oryctolagus cuniculus). J Exotic Pet Med. 2006; 2:145–149.

8. Cohen BH, Cosmetto AJ. The suture tension adjustment reel. A new device for the management of skin closure. J Dermatol Surg Oncol. 1992; 18:112–123.

9. Concannon MJ, Puckett CL. Wound coverage using modified tissue expansion. Plast Reconstr Surg. 1998; 102:377–384.

10. Freeman LJ, Pettit GD, Robinette JD, Lincoln JD, Person MW. Tissue reaction to suture material in the feline linea alba. A retrospective, prospective, and histologic study. Vet Surg. 1987; 16:440–445.

12. Gibson T, Kenedi RM. Biochemical properties of skin. Surg Clin North Am. 1967; 47:279–294.
13. Gillette RL, Swaim SF, Sartin EA, Bradley DM, Coolman SL. Effects of a bioactive glass on healing of closed skin wounds in dogs. Am J Vet Res. 2001; 62:1149–1153.

14. Hallock GG, Rice DC. Increased sensitivity in objective monitoring of tissue expansion. Plast Reconstr Surg. 1993; 91:217–222.

15. Hirshowitz B, Lindenbaum E, Har-Shai Y. A skin-stretching device for the harnessing of the viscoelastic properties of skin. Plast Reconstr Surg. 1993; 92:260–270.

16. Lam AC, Nguyen QH, Tahery DP, Cohen B, Sasaki GH, Moy RL. Decrease in skin-closing tension intraoperatively with suture tension adjustment reel, balloon expansion, and undermining. J Dermatol Surg Oncol. 1994; 20:368–371.

17. Lew D, Fuseler JW. The effect of pulsed expansion of subfascially placed expanders on the extent and duration of mitosis in the capsule and rat integument. J Oral Maxillofac Surg. 1993; 51:154–158.

18. Liang MD, Briggs P, Heckler FR, Futrell W. Presuturing-a new technique for closing large skin defects: clinical and experimental studies. Plast Reconstr Surg. 1988; 81:694–702.
19. Mackay DR, Saggers GC, Kotwal N, Manders EK. Stretching skin: undermining is more important than intraoperative expansion. Plast Reconstr Surg. 1990; 86:722–730.
20. Manning TO, Monteiro-Riviere NA, Bristol DG, Riviere JE. Cutaneous laser-Doppler velocimetry in nine animal species. Am J Vet Res. 1991; 52:1960–1964.
21. Melis P, Noorlander ML, Bos KE. Tension decrease during skin stretching in undermined versus not undermined skin: an experimental study in piglets. Plast Reconstr Surg. 2001; 107:1201–1205.

22. Molea G, Schonauer F, Blasi F. Progressive skin extension: clinical and histological evaluation of a modified procedure using Kirschner wires. Br J Plast Surg. 1999; 52:205–208.

23. Pavletic MM. Use of an external skin-stretching device for wound closure in dogs and cats. J Am Vet Med Assoc. 2000; 217:350–354.

24. Pavletic MM. Skin-stretching techniques. In : Pavletic MM, editor. Atlas of Small Animal Wound Management and Reconstructive Surgery. 3rd ed. Ames: Wiley-Blackwell;2010. p. 287–305.
25. Petro JA, Niazi ZBM. Immediate skin expansion: an old concept by a novel and inexpensive technique. Ann Plast Surg. 1996; 36:479–484.
26. Samis AJW, Davidson JSD. Skin-stretching device for intraoperative primary closure of radial forearm flap donor site. Plast Reconstr Surg. 2000; 105:698–702.

27. Spodnick GJ, Pavletic MM, Clark GN, Schelling SH, Kraus KH. Controlled tissue expansion in the distal extremities of dogs. Vet Surg. 1993; 22:436–443.

28. Timmenga EJ, Andreassen TT, Houthoff HJ, Klopper PJ. The effect of mechanical stress on healing skin wounds: an experimental study in rabbits using tissue expansion. Br J Plast Surg. 1991; 44:514–519.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download