Abstract
Canine hyperadrenocorticism (HAC) is one of the most common causes of general osteopenia. In this study, quantitative computed tomography (QCT) was used to compare the bone mineral densities (BMD) between 39 normal dogs and 8 dogs with HAC (6 pituitary-dependent hyperadrenocorticism [PDH]; pituitary dependent hyperadrenocorticism, 2 adrenal hyperadrenocorticism [ADH]; adrenal dependent hyperadrenocorticism) diagnosed through hormonal assay. A computed tomogaraphy scan of the 12th thoracic to 7th lumbar vertebra was performed and the region of interest was drawn in each trabecular and cortical bone. Mean Hounsfield unit values were converted to equivalent BMD with bone-density phantom by linear regression analysis. The converted mean trabecular BMDs were significantly lower than those of normal dogs. ADH dogs showed significantly lower BMDs at cortical bone than normal dogs. Mean trabecular BMDs of dogs with PDH using QCT were significantly lower than those of normal dogs, and both mean trabecular and cortical BMDs in dogs with ADH were significantly lower than those of normal dogs. Taken together, these findings indicate that QCT is useful to assess BMD in dogs with HAC.
Bone strength depends on the amount and density of bone [12]. Bone mineral density (BMD) can be used to evaluate skeletal status [18], and is one of the most important factors when considering bone strength and the risks associated with osteoporotic fractures [101131]. Therefore, measurement of bone mineral density is an important tool in the diagnostic evaluation of patients suspected of having or suffering from osteoporosis or other skeletal disorders [21]. In human medicine, it is preferable to measure only trabecular bone because it is thought to be eight times more sensitive to hormonal deficiencies and metabolic disease than cortical bone [61637].
The metabolic effects of canine hyperadrenocorticism (HAC) have long been recognized as the cause of several morphological changes with osteopenia that are most noticeable in vertebra [2833]. In human medicine, it has been reported that patients with primary adrenal hyperadrenocorticism (ADH) experienced more severe bone loss than those with pituitary-dependent hyperadrenocorticism (PDH) [27]. Subsequently, numerous studies have revealed that HAC and prolonged corticotherapy can cause osteopenia as well as osteoporosis, with increased frequency of pathological fractures [141823]. Based on these relationships, several techniques were developed for the noninvasive measurement of bone mineralization at various sites in humans, including dual X-ray absorptiometry (DXA) or quantitative computed tomography (QCT). However, DXA values are influenced by lack of homogeneity in soft-tissue composition and fat distribution, as well as by the conversion of 3-dimensional structures into a 2-dimensional image, which makes DXA susceptible to positioning errors [13163239]. In contrast, QCT is not subject to limitations related to positioning because it provides a three dimensional image of bone structure [32]. QCT also offers the advantage of selective measurement of the metabolically active and structurally important trabecular bone in the spine [15203436]. In human medicine, extensive literature exists on BMD measurement by QCT at the thoracic and lumbar vertebra. Moreover, the profile of age-related BMD assessments using QCT has already been established. In veterinary medical literature, one study of age-related changes and anatomic variations in trabecular BMD using QCT in normal cats has been conducted to date [8]. However, normal reference data for evaluation of disease-related changes in BMD is not available in dogs. Only QCT assessment of BMD changes associated with administration of prednisolone or prednisolone and alendronate sodium in dogs were studied [29]. Therefore, this study was conducted to establish normal BMD values of both trabecular and cortical bone in normal dogs and compare the results with the BMD of hyperadrenocorticism using QCT.
The acquisition of animal patients in this study was based on medical and diagnostic imaging records at the Chungbuk National University Veterinary Medical Center. Medical records from normal dogs that underwent abdominal computed tomogaraphy (CT) scans between August 2005 and October 2011 were searched, and 20 dogs with HAC that underwent scans between January 2011 and April 2012 were identified as meeting the criteria for inclusion in this study. Of the 20 dogs with HAC, eight (six with PDH and two with ADH) were included in this study following owner consent. The Institutional Animal Care and Use Committee at the Chungbuk National University approved all of the study procedures.
Thirty-nine normal dogs were selected based on their history of normal blood profiles, absence of significant findings on plain radiographs, and clinical BMD values that were within normal reference ranges. The diagnostic criteria for HAC were established based on clinical features including blood chemistry (Hitachi 7020 Autonomic Analyzer; Hitachi High-Technologies, Japan), urinalysis, plain abdominal radiograph (Kodak Directview CR500 system; Eastman Kodak, USA), ultrasonographic assessment of adrenal glands (ALOKA Prosound SSD-α5; Hitachi-Aloka, Japan), and hormonal analyses. Clinical diagnosis of HAC was based on signs of polyuria, polydipsia, polyphagia, abdominal distension, and dry hair coat with symmetrical abdominal alopecia, as well as thin skin that was sometimes hyper-pigmented. Blood chemistry analyses including alanine aminotransferase (ALT), alkaline phosphatase (ALP), and total cholesterol were also evaluated. In addition, urine specific gravity values < 1.015 were considered clinical indications in affected dogs. Hepatomegaly, including osteopenia on plain radiography and adrenomegaly defined by gland size greater than 5.9 mm, were also considered clinical manifestations of HAC. The final diagnosis of the eight HAC dogs was confirmed by measurement of a low dose dexamethasone suppression test (0.01 mg/kg, intravenously), or by a plasma adrenocorticotropic hormone response test (1.0 units/kg, intravenously). Exclusion criteria for the study were a history of generalized bone disease, metabolic or malignant disease related to the musculoskeletal system, trauma at the measurement sites, or any medical treatments that could influence bone metabolism. Dogs in the normal and HAC experimental groups were further subdivided by age based on previously established that the distinction between a full-grown adult and a dog in the geriatric stage is approximately 7 to 8 years [19]. Accordingly, dogs were subdivided into groups aged 7 to 8 years (Group 1) and dogs over 9 years old (Group 2) to determine if BMD changed significantly with age. Group 1 consisted of 20 normal and one PDH dog, while Group 2 contained 19 normal, five PDH, and two ADH dogs.
Of the eight HAC dogs, CT scans of one dog with PDH and two dogs with ADH were performed under general anesthesia. The CT scans of four dogs with PDH were performed while the dogs were awake, while one dog with PDH was scanned under mild sedation.
For both general anesthesia and sedation, each dog was premedicated with a subcutaneous injection of 0.05 mg/kg atropine (Atropine; Daewon Pharm, Korea). After endotracheal intubation, general anesthesia was induced by administration of an intravenous injection of 5 mg/kg propofol bolus (Anepol; Hana Pharm, Korea) and maintained using 2% to 2.5% isoflurane in oxygen (Isoflurane; Choongwae Pharm, Korea). During anesthesia, the oxygen saturation, heart rate, and end tidal CO2 levels were monitored continuously. An intramuscular injection of 5 mg/kg zolazepam with tiletamine (Zoletil; Virbac, France) was also administered under sedation. During the awakening period, two assistants wearing lead aprons, protective glasses, and gloves manually restrained the patients.
A GE HiSpeed CT/z (GE Healthcare, USA) scanner was used for quantitative measurement of BMD. Dogs were placed on the CT table in a ventral recumbency position. Based on lateral and dorsoventral scout views, transverse CT scans were performed at the mid-plane of the vertebra from the 12th thoracic to the 7th lumbar spine using a single 2 mm slice with 120 kVp, 100 mA, and a 512 × 521 voxel matrix.
All CT image data were imported to a PC workstation, and identification of the vertebral body from the 12th thoracic to the 7th lumbar spine was performed in each animal under a constant window (window width, 1000; window level, 200). As shown in Fig. 1, elliptical and manual traces of the region of interest (ROI), which consisted exclusively of the trabecular and cortical bones of the vertebral body, respectively, were drawn using the eFilm viewing software (eFilm Workstation ver. 3.0.1; Merge, USA) and the OsiriX-DICOM viewer (OsiriX 64-bit extension; Pixmeo, Switzerland). For each ROI, the mean Hounsfield units (HU) values were calculated and recorded. To evaluate inter-observer variations, measurement of the ROI values was repeated by three radiologists. To convert the HU values to BMDs, a bone mineral reference CIRS phantom (Computerized Imaging Reference System, USA) was scanned using the same parameters applied when performing scans of the dogs (panel A in Fig. 2). The phantoms contained calibration objects with equivalent densities of 50, 100, and 150 mg/cm3 calcium hydroxyapatite. The mean HU values were converted to BMD (mg/cm3) using a phantom-derived linear regression equation (panel B in Fig. 2) as previously described [57].
Statistical analyses of data were performed using a statistical software program for Windows (SPSS ver. 12.0; SPSS, USA). Differences in the mean BMDs between normal and HAC patients were analyzed using a two-tailed t-test. Similarly, one-way analysis of variance (ANOVA) was used to determine significant differences between mean BMDs among groups (normal, PDH, and ADH). Differences were evaluated by Bonferroni's test. In addition, Pearson's correlation analysis was conducted to determine correlations between mean BMD values and ages in the normal group. The level of statistical significance used was 0.05 for all tests. All data were expressed as the mean ± standard deviation (SD).
In the current study, the breeds in the normal dog group included Maltese (n = 8), mixed breed (n = 7), Dachshund (n = 5), Schnauzer (n = 4), Shih-Tzu (n = 3), Beagle (n = 2), Cocker spaniel (n = 2), Pomeranian (n = 2), Yorkshire terrier (n = 2), Poodle (n = 2), Pekingese (n = 1), and Alaskan malamute (n = 1). There were 16 male dogs, two of which were neutered, and 20 female dogs, one of which was spayed. The mean age (years) for each group was 7.4 ± 0.5 and 11.6 ± 2.9, respectively, and the mean body weight (kg) was 7.23 ± 3.1 and 8.1 ± 5.7 for Groups 1 and 2, respectively.
The breed, age, sex, body weight, serum profiles, hormonal data, and clinical signs for the HAC group are indicated in Table 1. Six of the eight dogs were first diagnosed at our hospital, while two (dogs 2 and 4) presented from regional hospitals with a definitive diagnosis of HAC. The breeds of HAC dogs were included in the normal dog group, and thus the breed distribution between the normal and HAC dogs was generally consistent. The ages of all HAC patients fell within the ranges defined as adult or geriatric. All patients exhibited a minimum of two clinical indications related to HAC. The most common clinical signs initially reported by owners of the eight dogs with HAC were polyuria (n = 8), polydipsia (n = 8), dermatological abnormalities (e.g., alopecia, thin skin, or hyperpigmentation (n = 7), polyphagia (n = 5), and panting (n = 2)). However, there was no singularity in any of the clinical laboratory tests. Evaluation of the diagnostic imaging data revealed that seven of the eight HAC dogs had hepatomegaly and osteopenia. In addition, bilateral adrenomegaly (defined as a gland ≥ 0.59 cm long with adrenal thickness at the caudal polar width) was identified in three dogs except for two ADH patients with distinctive unilateral adrenomegaly, unilateral adrenomegaly or normal size of adrenal glands were also seen. Table 2 shows the treatment period of HAC patients and the association among plasma cortisol concentrations as determined by the ACTH hormonal test.
The Kappa (κ) values of interobserver agreement in normal, PDH, and ADH dogs were 0.786, 0.639, and 0.807, respectively. In addition, κ = 0.744 (normal and PDH) and κ = 0.897 (normal and ADH); therefore, there was a statistically substantial agreement among BMD measurements by the three radiologists.
The mean BMDs (± SD) of each vertebra in age-subdivided groups (Groups 1 and 2) of normal dogs are described in Table 2. A statistically negative correlation between BMDs and age was observed. The mean BMDs of trabecular bone (tBMD) and cortical bone (cBMD) decreased significantly with age (p < 0.05), except for T12 and T13 at the cortical bone. However, the mean BMD value of both trabecular and cortical bone at each vertebra showed no gender difference.
In HAC dogs, the mean BMDs (mg/cm3) for the 12th thoracic to 7th lumbar vertebra for both PDH and ADH dogs at trabecular and cortical bone were compared with those of normal dogs. ANOVA revealed that the mean tBMDs differed significantly between normal and HAC patients in the same age groups (p < 0.001-0.002) (Fig. 3). Moreover, the mean cBMDs were lower than those of normal dogs. However, only the T13, L1, L2, L4, L5, and L6 level differed significantly (p < 0.002-0.033). HAC dogs were subdivided into PDH and ADH groups and then compared with normal dogs (Fig. 4). The tBMDs of PDH patients showed lower distribution than those of normal patients (p < 0.001-0.05), and the tBMD values of ADH (p < 0.001). There were no significant differences in cBMDs between PDH patients and normal dogs except for at the L4 (p = 0.48) and L5 (p = 0.28) vertebrae. The cBMDs of ADH patients were significantly lower (p < 0.001-0.002) than those of normal patients. The relationship between circulating cortisol concentrations and BMDs was illustrated at both L2 and L5 (Fig. 5). There was a significantly negative association between cortisol concentrations and BMDs in both trabecular and cortical bone at that site (p < 0.001). The relationship between BMDs and chronological age was also investigated by scatter plots of the data for each vertebra (Figs. 6 and 7). In trabecular bone, a statistically negative correlation between trabecular bone mineral density and age was confirmed. In addition, scatter plots showed that tBMDs of HAC patients had lower distribution than those of normal patients. In cortical bone, scatter plots showed that cBMDs of PDH patients were not significantly different from cBMDs of normal patients. However, cBMDs of ADH patients were definitely different than those of normal patients.
Using predicted linear regression based on scatter plots of normal BMDs at both trabecular and cortical bone, actual tBMDs and cBMDs of HAC patients were converted into percentages (Figs. 8 and 9). tBMDs of both PDH and ADH patients were approximately 20% and 40% lower than those of normal patients at each vertebra, respectively. cBMDs of PDH patients were also about 10% reduced and rather to be increased at L6 vertebra. However, the cBMDs of ADH patients were approximately 60% lower than those of normal dogs, and the difference between ADH and PDH patients at this site was quite evident.
The present study quantitatively evaluated the BMD of HAC dogs by QCT and confirmed that HAC is associated with lower tBMDs and cBMDs at anatomical sites from the 12th thoracic to the 7th lumbar vertebra relative to normal dogs.
In this study, BMD was calculated from the HU value of the phantom containing mineral equivalents of known calcium density based on linear regression. A positive linear correlation between HU and bone calcium concentrations (mg/cm3) was determined using human cadaveric lumbar vertebra [5]. The modified method involving the phantom was first applied in 1980 [7]. In that study, the authors reported that the major problems influencing QCT precision included scanner related changes that occurred with time (e.g., X-ray tube aging, detector drifts, and heating), as well as patient repositioning and the subsequent lack of homogeneity within the vertebral body. To correct these problems, the authors suggested calibration of the CT scan using the phantom. A separate study verified that application of the linear regression phantom method reduced the calcium concentration variability, and yielded a precision, or coefficient of variation, of 0.1% to 3.0% [122535]. Therefore, we converted the HU values to BMD using the calibration phantom-derived equation.
The slice thickness and the size of ROI are critical factors influencing the precision of CT measurements. CT scans were performed using a 2 mm slice thickness in the present study. When a thinner slice is used, focal variation can strongly affect the radiopacities in images because QCT analyzes the mean of radiopacities of the inner region of the slice volume [22]. However, in a recent study, there were no significant differences in BMD based on the means of QCT images with slice thicknesses of 2.4 mm, 4.8 mm, and 9.6 mm, and thinner CT images could be used in toy- or small-breed dogs if accompanied by the appropriate ROI size and CT image selection [3].
In the present study, the measurement sites for BMD assessment were from the 12th thoracic to the 7th lumbar vertebra. These sites were selected because the vertebrae, pelvis, and hips are skeletal sites rich in trabecular bone [30]. In particular, the lumbar spine was reported to be more susceptible to the detrimental effects of endogenous glucocorticoids than any other sites [27]. The pelvis and hips were not considered because the owners of the four dogs with PDH did not consent to general anesthesia or sedation, and the position of the dogs during the CT scan was not proper for scanning those sites when limited by manual restraint. Therefore, further studies are required to measure the BMD of the pelvis, hip, and femur.
Our results indicated that both tBMD and cBMD in normal dogs at each vertebra had a tendency to decrease with age (p < 0.05). In humans, the profile of bone development was reported to increase in mineral content and density until approximately age 35, with gradual loss thereafter [17]. In veterinary medicine, tibial bone density in beagle dogs increased until age six, then declined [24], which was in accordance with the results of a prior human study that indicated age is very important when considering bone strength.
In the present study, both normal dogs and dogs with HAC were composed of various breeds. These findings indicate that dogs have a variety of vertebral sizes according to breed and body size, and that differences in the BMD between normal and HAC groups could be affected in this study. However, no studies have investigated the effects of breed and size of dogs on BMD. Accordingly, further BMD assessments due to breed and body size variation of dogs is needed.
Additionally, no statistically significant differences in mean BMD values were detected in the gender-related normal group at any of the vertebra. Numerous human studies have documented bone loss in women following menopause. However, in veterinary medicine, only one study has shown age-related trabecular bone loss in both male and female beagles, and very little change in bone mineral occurred in either gender [24]. Accordingly, our results were in agreement with prior reports of bone loss in beagles. One theory regarding the difference between humans and dogs is that, unlike humans, dogs do not experience menopause. Consequently, decreased BMD was not noted in normal female dogs.
In the present study, the difference in BMD between healthy dogs and dogs with HAC was evident in the trabecular bone of each vertebra. In addition, the BMD of healthy dogs and those with ADH was only distinctly different in the cortical bone. These findings confirm that significant demineralization of the tBMD occurred in HAC patients. Trabecular bone, which exists as a lattice, divides the interior volume of the bone into intercommunication pores that are filled with a variable mixture of red and yellow marrow [9]. Given its morphophysiological characteristics, trabecular bone could more accurately reflect the HAC-induced BMD changes than cortical bone, which would explain why cortical bone has attracted less attention in BMD research. For this reason, trabecular bone is considered the anatomical site for BMD assessments in the spine. In addition, patients presenting with PDH or ADH have different patterns of adrenal steroid secretion. Although it is currently unknown whether these HAC variants have different potential to induce osteopenia [27], reports have indicated that, unlike PDH, ADH results in the exclusive secretion of cortisol, which produces a pure hypercortisolism [4]. For this reason, differences between PDH and ADH are believed to result in significantly lower BMD in ADH patients than the BMD observed in normal or PDH patients, regardless of trabecular or cortical bone.
The initial plasma cortisol profiles were compared with the BMDs of L2 and L5. Both L2 and L5 were selected as anatomical sites because of their higher significant difference in BMD compared to other vertebra. The data indicated that plasma cortisol concentrations were negatively associated with the loss of BMD. These observations suggest that the endogenous cortisol profile is likely another determinant of bone density, and could influence the risk of osteopenia in HAC dogs. Therefore, serial assessment of plasma cortisol in HAC dogs could be a useful marker of HAC.
In humans, Z-scores (patient value - mean of the normal value corresponding to sex and age group/standard deviation of the values of the normal group) were used to assess how patient values deviated from the average normal value [26]. However, the present study included only 39 normal dogs, and they did not represent the normal references of BMDs. Thus, the Z-score value was not directly applicable to our study. To determine the decline in HAC patient BMDs, a predicted linear regression equation based on scatter plots of normal BMDs was used to determine if BMDs in HAC dogs were lower than those of normal dogs of the same age based on the assumption that normal values at each vertebra were 100%. Further, actual tBMDs and cBMDs of HAC patients were converted to a percentage, which revealed that the values of tBMDs and cBMDs of both PDH and ADH patients were approximately 20%, 40%, 10%, and 60% less than those of normal patient values, respectively, further supporting the aforementioned comments.
It should be noted that there were several limitations to the current study. First, the number of subjects was small. Considering the nature of HAC disease, consent of the owners for performance of the CT procedure was not easily obtained. Second, the BMDs measured in this study could have inherent measurement errors. The vertebral body is not a homogeneous structure. In addition, the heterogeneous distribution of the trabecular bone may result in errors when selecting the ROI. In humans, automated software that locates the mid-portion of each vertebral body is used to select the ROI [38]. However, in this study, although the inter-observer variation indicated agreement, manual tracing of the ROI introduced additional limitations. Additionally, because of the retrospective nature of this study, the CT scanning parameters used on the normal dogs were not standardized to each patient, and the dates of CT scans in the PDH dogs differed. Before HAC diagnosis and after initiation of treatment, both the duration and severity of the illness differ. Finally, the BMDs may be different; therefore, the values calculated in the present study might not reflect the actual BMDs.
Several canine breeds of varying age, sex, and body weight will be included in future studies. In addition, both tBMDs and cBMDs of HAC patients will be compared before and after treatments. Following a human study in 2004, Mancini et al. reported that the bone demineralization that occurs with HAC can be reversed after the patient is cured, but the recovery of bone loss is gradual and can take approximately ten years [24]. Consequently, special attention should be paid to canine patients with HAC, and attempts should be made to establish adequate treatments.
The mean tBMDs of vertebra in PDH dogs measured by QCT were significantly lower than those of normal dogs, and both mean trabecular and cortical BMDs of vertebra in ADH dogs were significantly lower than those of normal dogs. Therefore, we conclude that QCT is useful to assess BMD in dogs with HAC.
Figures and Tables
Fig. 1
Region of interest (ROI) selection for measuring Hounsfield units (HU) value at both trabecular bone (A) and cortical bone (B) of the 12th thoracic to 7th lumbar vertebra. An elliptical and manual trace of ROI that consisted exclusively of trabecular and cortical bone of the vertebral body, respectively, were drawn.

Fig. 2
The method of conversion of the Hounsfield units (HU) value to bone mineral density (BMD). Phantoms that contain known concentrations of calcium hydroxyapatite (from left to right 50, 100, 150 mg/cm3) were scanned with the same CT scan parameters (A). The mean and standard deviation of the HU values in ROI were measured within each calibration phantom image, after which measurements of the HU values were transformed into calcium hydroxyapatite concentration (mg/cm3) using the corresponding slope and the linear regression line (B).
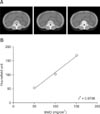
Fig. 3
Comparison of bone mineral density between normal and HAC dogs at trabecular and cortical bone. Box-and-whisker plots show significantly lower tBMDs of HAC patients than those of normal dogs (A). Significant differences were only observed in T13, L1, L2 L4, L5, and L6. The central boxes represent values from the lower to upper quartile (25th to 75th percentile). The middle line represents the median. Significant differences were determined by a t-test. *p < 0.05, **p < 0.01, ***p < 0.001.

Fig. 4
Comparison of bone mineral density among each group with the same ages. Box-and-whisker plots show significantly lower tBMDs of hyperadrenocorticism (HAC) patients than those of normal dogs (A). There was no significant difference between the cBMD of normal and pituitary dependent hyperadrenocorticism (PDH) patients (B). However adrenal dependent hyperadrenocorticism (ADH) patients showed significantly lower cBMD values than normal patients. The central boxes represent values from the lower to the upper quartile (25th to 75th percentile). The middle line represents the median. Significant differences were determined by one way ANOVA. *p < 0.05, **p < 0.01, ***p < 0.001.

Fig. 5
Relationship between characteristics of cortisol concentration and bone mineral density at L2 and L5 among HAC dogs. Both L2 and L5 were selected as anatomical sites because they showed higher significant differences than any other sites. There were negative associations between cortisol concentration and bone mineral density of both trabecular and cortical bone at L2 and L5. Significant differences were determined by a t-test.

Fig. 6
Relationship between trabecular bone mineral densities (tBMDs) and chronological age among normal, PDH, and ADH patients at each vertebra. Predicted linear regression lines were developed and plotted along with scatter from actual data of tBMDs. The tBMDs-to age relationship was significant (R2 = 0.02-0.3, p < 0.01-0.001). The statistically negative correlation between trabecular bone mineral density and age was confirmed. In addition, scatterplots showed that the tBMDs of HAC patients were lower than those of normal patients.

Fig. 7
Relationship between cortical bone mineral densities (cBMDs) and chronological age among normal, PDH, and ADH patients at each vertebra. Predicted linear regression lines were developed and plotted along with scatter from actual data of cBMDs. The cBMDs-to age relationship was significant (R2 = 0.02-0.12, p < 0.01-0.001). The statistically negative correlation between cortical bone mineral density and age was confirmed. Scatterplots also show that cBMDs of PDH patients were not significantly different from cBMDs of normal patients. However cBMDs of ADH patients are definitely different from those of normal patients.

Fig. 8
The percentage of trabecular bone mineral densities (tBMD) of HAC dogs compared with those of normal dogs. Linear regression equations obtained from scatter plots of normal BMDs were used to see if BMDs of HAC dogs were actually lower than those of normal dogs at the same ages, assuming normal values are 100%. The predicted age-adjusted tBMDs of PDH and ADH dogs were approximately 20% and 40% less than those of normal patients at each vertebra, respectively.

Fig. 9
The percentage of cortical bone mineral densities (cBMDs) of HAC dogs compared with that of normal dogs. Linear regression equations obtained from scatter plots of normal BMDs were used to see if BMDs of HAC dogs were actually lower than those of normal dogs at the same ages, assuming normal values are 100%. The predicted age-adjusted cBMDs of PDH patients were about 10% reduced and rather to be increased at L6 vertebra. However, the cBMDs of ADH patients were approximately 60% lower than those of normal dogs, and the difference between ADH and PDH patients at this site was quite evident.

Table 1
Signalments, serum profiles, hormonal data, and clinical signs of dogs with hyperadrenocorticism

Table 2
Mean bone mineral density of age-divided normal groups at each vertebral level

*Age-divided groups (Group 1, 7-8 years; Group 2, > 9 years). Data are the mean values ± the standard deviation. There was a statistically significant difference between the mean BMD value with age in both trabecular and cortical bone (p < 0.05). However, the mean BMD of T12 and T13 in cortical bone did not differ significantly. The p values were determined by Pearson's correlation.
Acknowledgments
This work was supported in part by a grant from the National Research Foundation of Korea funded by the Korean Government (NRF-2013R1A2A2A04008751), Korea.
References
1. Aerssens J, Boonen S, Joly J, Dequeker J. Variations in trabecular bone composition with anatomical site and age: potential implications for bone quality assessment. J Endocrinol. 1997; 155:411–421.

2. Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003; 14:Suppl 3. S13–S18.

3. Bae Y, Park S, Jeon S, Lee G, Choi J. Effect of region of interest and slice thickness on vertebral bone mineral density measured by use of quantitative computed tomography in dogs. Am J Vet Res. 2014; 75:642–647.

4. Bertagna C, Orth DN. Clinical and laboratory findings and results of therapy in 58 patients with adrenocortical tumors admitted to a single medical center (1951 to 1978). Am J Med. 1981; 71:855–875.

5. Bradley JG, Huang HK, Ledley RS. Evaluation of calcium concentration in bones from CT scans. Radiology. 1978; 128:103–107.

6. Braillon PM. Quantitative computed tomography precision and accuracy for long-term follow-up of bone mineral density measurement: a five year in vitro assessment. J Clin Densitom. 2002; 5:259–266.

7. Cann CE, Genant HK. Precise measurement of vertebral mineral content using computed tomography. J Comput Assist Tomogr. 1980; 4:493–500.

8. Cheon H, Choi W, Lee Y, Lee D, Kim J, Kang JH, Na K, Chang J, Chang D. Assessment of trabecular bone mineral density using quantitative computed tomography in normal cats. J Vet Med Sci. 2012; 74:1461–1467.

9. Dyson ED, Jackson CK, Whitehouse WJ. Scanning electron microscope studies of human trabecular bone. Nature. 1970; 225:957–959.

10. Ebbesen EN, Thomsen JS, Beck-Nielsen H, Nepper-Rasmussen HJ, Mosekilde L. Lumbar vertebral body compressive strength evaluated by dual-energy X-ray absorptiometry, quantitative computed tomography, and ashing. Bone. 1999; 25:713–724.

11. Faulkner KG, Glüer CC, Majumdar S, Lang P, Engelke K, Genant HK. Noninvasive measurements of bone mass, structure, and strength: current methods and experimental techniques. AJR Am J Roentgenol. 1991; 157:1229–1237.

12. Faulkner KG, Glüer CC, Grampp S, Genant HK. Crosscalibration of liquid and solid QCT calibration standards: corrections to the UCSF normative data. Osteoporos Int. 1993; 3:36–42.

13. Formica C, Loro ML, Gilsanz V, Seeman E. Inhomogeneity in body fat distribution may result inaccuracy in the measurement of vertebral bone mass. J Bone Miner Res. 1995; 10:1504–1511.

14. Füto L, Toke J, Patócs A, Szappanos A, Varga I, Gláz E, Tulassay Z, Rácz K, Tóth K. Skeletal differences in bone mineral area and content before and after cure of endogenous Cushing's syndrome. Osteoporos Int. 2008; 19:941–949.

15. Genant HK, Cann CE, Pozzi-Mucelli RS, Kanter AS. Vertebral mineral determination by quantitative CT: clinical feasibility and normative data. J Comput Assist Tomogr. 1983; 7:554.
16. Gilsanz V, Perez FJ, Campbell PP, Dorey FJ, Lee DC, Wren TAL. Quantitative CT reference values for vertebral trabecular bone density in children and young adults. Radiology. 2009; 250:222–227.

17. Goldsmith NF, Johnston JO, Picetti G, Garcia C. Bone mineral in the radius and vertebral osteoporosis in an insured population. A correlative study using 125I photon absorption and miniature roentgenography. J Bone Joint Surg Am. 1973; 55:1276–1293.

18. Grampp S, Jergas M, Lang P, Steiner E, Fuerst T, Glüer CC, Mantur A, Genant HK. Quantitative CT assessment of the lumbar spine and radius in patients with osteoporosis. AJR Am Roentgenol. 1996; 167:133–140.

19. Hand MS, Zicker SC, Norvotny BJ. Feeding young adult dogs: before middle age and older. In : Hand MS, Zicker SC, Norvotny BJ, editors. Small Animal Clinical Nutrition Quick Consult. 1st ed. Topeka: Mark Morris Institute;2011. p. 1–4.
20. Jergas M, Genant H. Current methods and recent advances in the diagnosis of osteoporosis. Arthritis Rheum. 1993; 36:1649–1662.

21. Kalender WA, Fischer M. Quality control and standardisation of absorptiometric and computer tomographic measurements of bone mineral mass and density. Radiat Prot Dosimetry. 1993; 49:229–233.

22. Lee JY, Kim KD, Park CS. Effect of the slice thickness and the size of region of interest on CT number. Korean J Oral Maxillofac Radiol. 2001; 31:85–91.
23. Mancini T, Doga M, Mazziotti G, Giustina A. Cushing's syndrome and bone. Pituitary. 2004; 7:249–252.

24. Martin RK, Albright JP, Jee WS, Taylor GN, Clarke WR. Bone loss in the beagle tibia: influence of age, weight, and sex. Calcif Tissue Int. 1981; 33:233–238.

25. McCollough CH, Kaufmann RB, Cameron BM, Katz DJ, Sheedy PF 2nd, Peyser PA. Electron-beam CT: use of a calibration phantom to reduce variability in calcium quantitation. Radiology. 1995; 196:159–165.

26. Mei Z, Grummer-Strawn LM. Standard deviation of anthropometric Z-scores as a data quality assessment tool using the 2006 WHO growth standards: a cross country analysis. Bull World Health Organ. 2007; 85:441–448.

27. Minetto M, Reimondo G, Osella G, Ventura M, Angeli A, Terzolo M. Bone loss is more severe in primary adrenal than in pituitary-dependent Cushing's syndrome. Osteoporos Int. 2004; 15:855–861.

28. Norrdin RW, Carpenter TR, Hamilton BF, Brewster RD. Trabecular bone morphometry in beagles with hyperadrenocorticism and adrenal adenomas. Vet Pathol. 1988; 25:256–264.

29. Park S, Oh J, Son KY, Cho KO, Choi J. Quantitative computed tomographic assessment of bone mineral density changes associated with administration of prednisolone or prednisolone and alendronate sodium in dogs. Am J Vet Res. 2015; 76:28–34.

30. Peloschek P, Grampp S. Vertebral fractures and osteoporosis. In : Cassar-Pullicino VN, Imhof H, editors. Spinal Trauma-An Imaging Approach. 1st ed. New York: Theime Medical;2006. p. 203–211.
31. Ross PD, Davis JW, Epstein RS, Wasnich RD. Pre-existing fractures and bone mass predict vertebral fractures incidence in women. Ann Intern Med. 1991; 114:919–923.

32. Schneider S, Breit SM, Grampp S, Künzel WW, Liesegang A, Mayrhofer E, Zentek J. Comparative assessment of bone mineral measurements obtained by use of dual-energy X-ray absorptiometry, peripheral quantitative computed tomography, and chemical-physical analysis in femur of juvenile and adult dogs. Am J Vet Res. 2004; 65:891–900.

33. Schwarz T, Störk CK, Mellor D, Sullivan M. Osteopenia and other radiographic signs in canine hyperadrenocorticism. J Small Anim Pract. 2000; 41:491–495.

34. Steiger P, Block JE, Steiger S, Heuck AF, Friedlander A, Ettinger B, Harris ST, Glüer CC, Genant HK. Spinal bone mineral density measured with quantitative CT: effect of region of interest, vertebral level, and technique. Radiology. 1990; 175:537–543.

35. Suzuki S, Okumura H, Yamamuro T, Yamamoto I. Bone mineral measurement by CT apparatus with simultaneous use of reference phantom: error factor and clinical evaluation. J Bone Miner Metab. 1988; 6:18–25.

36. Theodorou DJ, Theodorou SJ, André MP, Kubota D, Weigert JM, Sartoris DJ. Quantitative computed tomography of spine: comparison of three-dimensional and two-dimensional imaging approaches in clinical practice. J Clin Densitom. 2001; 4:57–62.
37. Van Berkum FNR, Birkenhäger JC, Zeelenberg J, Pols HAP, Birkenhäger-Frenkel DH, van Veen LCP, Trouerbach WT, Stijnen T. Noninvasive axial and peripheral assessment of bone mineral content: a comparison between osteoporotic women and normal subjects. J Bone Miner Res. 1989; 4:679–685.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download