Abstract
Monoclonal anti-enrofloxacin antibody was prepared for a direct competitive enzyme-linked immunosorbent assay (ELISA) and purification system using monoclonal antibody (mAb) coupled magnetic nanoparticles (MNPs). The IC50 values of the developed mAb for enrofloxacin (ENR), ciprofloxacin, difloxacin, sarafloxacin, pefloxacin, and norfloxacin were 5.0, 8.3, 9.7, 21.7, 36.0, and 63.7 ng/mL, respectively. The lowest detectable level of ENR was 0.7 ng/mL in the prepared ELISA system. To validate the developed ELISA in the food matrix, known amounts of ENR were spiked in meat and egg samples at 10, 20 and 30 ng/mL. Recoveries for ENR ranged from 72.9 to 113.16% with a coefficient of variation (CV) of 2.42 to 10.11%. The applicability of the mAb-MNP system was verified by testing the recoveries for ENR residue in three different matrices. Recoveries for ENR ranged from 75.16 to 86.36%, while the CV ranged from 5.08 to 11.53%. Overall, ENR-specific monoclonal antibody was prepared and developed for use in competitive to ELISAs for the detection of ENR in animal meat samples. Furthermore, we suggest that a purification system for ENR using mAb-coupled MNPs could be useful for determination of ENR residue in food.
Fluoroquinolones (FQs) have been widely used as human and veterinary drugs, especially for the prevention and treatment of various infectious diseases in domestic animals, poultry, and fish [21]. FQs act through inhibition of DNA-gyrase, abolishing activity by interfering with the DNA rejoining reaction [314]. The widespread use of FQs has led to contaminating residues in foodstuffs derived from treated animals, which can induce unwanted reactions such as erythema, burning, and itching in humans and animals [25]. Furthermore, antibiotics released into the natural ecosystem can modify the local environmental microbiota by changing the composition or activity [123]. Many regulatory agencies have established a maximum residue limit for FQs in milk, meat, and other foods [56]. For example, the maximum sum of enrofloxacin (ENR) and its metabolite ciprofloxacin in muscle was set at 100 µg/kg for all animal species in the European Union [14].
Conventional methods such as liquid chromatography coupled to various detectors including ultra-violet (UV), mass spectrometry, or fluorescence detection are used for detection of drug residues [231]. These techniques have been shown to be highly specific and sensitive, but such traditional methods require expensive equipment and interpretation of complicated chromatograms or spectral results [13]. Therefore, a rapid, reliable, and easy screening method is required for monitoring of large samples [4]. Enzyme-linked immunosorbent assay (ELISA), which is based on specific antigen-antibody interactions, is the most suitable method for rapid screening of ENR residue in the veterinary field [2930]. Monoclonal or polyclonal antibodies have been developed for use in immunochemical detection assays [920]. Many organic solvents or immunoaffinity columns are required to separate FQs from the matrix to enable their analysis. The magnetic nanoparticle (MNP) has emerged for various applications such as gene and drug delivery, treatment of disease, and diagnosis [1124]. MNP can bind to different functional groups such as oligonucleotide probes, antibodies, and proteins to produce nanoprobes [19]. Previous studies have indicated the usefulness of nanoparticles for identification of pathogenic bacteria in DNA-microarrays, isolating target organisms from food matrices and screening metal ions in water [81726]. Additionally, we reported a rapid purification method using monoclonal antibodies against mycotoxin and MNPs [18].
This study was conducted to develop a direct competitive ELISA system to screen for ENR in foodstuffs and to develop a purification tool for isolating ENR by utilizing the ENR monoclonal antibody (mAb) and MNPs.
Bovine serum albumin (BSA), enrofloxacin, ciprofloxacin, difloxacin, sarafloxacin, pefloxacin, norfloxaicin, keyhole limpet hemocyanin (KLH), N-hydroxysuccinimide (NHS), triethylamine, carbonate-bicarbonate buffer, Tween 20, glutaraldehyde solution (Grade II, 25%), glycine, Freund's complete adjuvant/incomplete adjuvant, and 1-ethyl-3-(dimethylaminopropyl)carbodiimide hydrochloride (EDC) were purchased from Sigma-Aldrich (USA). Goat anti-mouse IgG was purchased from Abcam (UK). An EZ-Link Plus Activated Peroxidase kit was purchased from Thermo Scientific (USA). 3, 3', 5, 5'-tetra-methylbenzidine (TMB) solution was purchased from KPL (USA). Amine-functionalized MNPs (160 nm) were acquired from Nanobric (Korea). A HiTrap Protein G HP kit was obtained from GE Healthcare (UK).
Five female BALB/c mice (6-week old) were purchased from Orient Bio Incorporated (Korea). The animal room was maintained at 22 ± 2℃ (relative humidity, 50% ± 10%) and illuminated using a 12-h day/12-h night cycle. The experiments were performed in accordance with the Code of Laboratory Animal Welfare Ethics, National Research and Quarantine Service, Korea (NVRQS).Experimental design was approved by the NVRQS Animal Welfare Committee.
The immunogen ENR-KLH and coating antigen ENR-BSA were prepared using the NHS ester method as previously described, with slight modifications [15]. In this procedure, a total of 4 mg of ENR (0.011 mmol) was dissolved in DMF (35 µL) and added to 0.25 MNHS solution (40 µL) and EDC (40 mg, 0.258 mmol) with continuous stirring. The mixture was then incubated for 24 h at 4℃ without light, after which 2.5 mL of coupling buffer with 10% (w/w) KLH or BSA were slowly added to the mixture solution while stirring, followed by 6 h of incubation at 4℃ without light. Finally, the reaction mixture was dialyzed (molecular weight cut-off : 10,000 Da) against PBS (0.1 mol/L, pH 7.4) at 4℃ and stored at -20℃ until use as the immunogen. The UV absorbance method was used to determine if the conjugate was a success.
Mice were immunized subcutaneously with 100 µg of ENR-KLH conjugate five times with a 2-week interval between immunizations. The initial injection of ENR-KLH conjugate was administered in an equal volume of Freund's complete adjuvant, while the following four injections were administered using Freund's incomplete adjuvant. Animals were boosted with 100 µg of the same conjugate in PBS.
The cell fusion protocol was based on a previously described standard method [27], with slight modification. The mouse that had the highest polyclonal antibody titer was sacrificed, after which spleen cells were mixed with SP2/O myeloma cells using polyethylene glycol 1500 (Roche, Germany). The ratio of spleen cells from the immunized mouse fused with SP2/O myeloma cells was about 5 : 1. Fused cells were grown in HAT selection medium, plated in 96-well microculture plates, and hybridoma supernatants were then screened for specific antibodies using indirect competitive ELISA.
To produce the ascites fluid, hybridoma cells in medium were intraperitoneally injected into a mature female BALB/c mouse that had previously been injected with 0.5 mL of pristine. After 14 days, the ascites fluid was collected, and monoclonal IgG from ascites was purified using Protein G column chromatography (GE Healthcare). The isotype of the purified antibody was determined using a mouse mAb isotyping kit (Roche) according to the manufacturer's instructions. The ENR mAb was conjugated to horseradish peroxidase (HRP) using an HRP labeling kit (Thermo Scientific), then stored at -20℃ until use.
The presence and specificity of antibodies were tested by indirect, competitive ELISA as described below. The microplates were coated with ENR-BSA dissolved in 0.5 M carbonate buffer (100 ng per well, 100 µL). Following overnight incubation at 4℃, plates were washed three times with washing buffer and blocked with 1% casein in PBS (w/v, 250 µL/well), after which they were incubated for 1 h at 37℃. The blocking solution was then removed, after which PBS buffer or competitor in PBS buffer (80 µL/well) was added to each well, followed by the addition of serum or culture supernatants(80 µL/well). Samples were then incubated at room temperature for 1 h, after which they were washed three times, and 100 µL of anti-mouse IgG- HRP conjugate (dilution 1 : 1000 in 1% skim milk) was added and incubated for 1 h. Each well was washed with washing buffer and 100 µL of TMB solution for 5 min, after which color development was halted by adding 100 µL of 2N H2SO4, and the absorbance at 450 nm was measured using a microtiter plate reader.
Antigen coating of the microplate and blocking were carried out as described above. After removal of the blocking solution, standard ENR or sample ENR (80 µL/well) were added to each well, followed by the addition of ENR antibody-HRP conjugate (80 µL/well) and incubation at room temperature for 1 h. After washing three times, color development was conducted as described in the indirect competitive ELISA screening procedure.
Homogenized chicken muscle, egg, and cattle muscle were weighed (1 g) into 50 mL conical tubes, after which the samples were spiked (100 µL) with different concentrations of ENR (10, 20, 25, 30, 50 and 100 ng/g). Five mL of PBS buffer were then added, and the mixture was agitated on a shaker for 10 min. The samples were subsequently centrifuged at 3,000 × g for 20 min at 4℃, after which the supernatants were added directly to the microtiter plates and analyzed using direct competitive ELISA systems.
A total of 3 mg of an amine-functionalized MNP suspension (100 µL) was washed three times using a magnet in coupling buffer (0.01 M pyridine, pH 6.0), after which a 1 mL solution of 5% glutaraldehyde was added. Following incubation for 30 min at room temperature while shaking, the particles were washed with coupling buffer by magnetic separation. Next, mAb coupling with the MNPs was conducted by dissolving 130 µg of ENR mAb in 500 µL of coupling buffer. The mixture was subsequently rotated at 94 × g overnight at room temperature, after which the MNP-mAb conjugates were washed with coupling buffer (500 µL). The reaction was quenched by the addition of a solution of glycine and then washed three times with PBS buffer and stored in PBS buffer at 4℃ before use.
Sample preparation was carried out as described above. MNP-mAb conjugates were added to 500 µL of the processed samples for 5 min at room temperature while shaking. After the reaction solution was magnetically removed from the supernatant using a magnet, which was discarded, ENR was dissociated from the MNP-mAb conjugatesby adding 100% methanol (500 µL) with gentle shaking. Following dissociation, the MNP-mAb conjugates were magnetically isolated, and levels of ENR in the supernatant were measured using HPLC.
Enrofloxacin separated by MNP-mAb conjugates was detected via HPLC using an XTerra RP18 column (Waters, Ireland). The chromatographic column was then equilibrated with a mixture of water-acetonitrile-methanol (800 : 170 : 30; v/v/v). The flow rate of the mobile phase was 1.2 mL/min. Dissociated samples (20 µL) were injected into the HPLC system (Waters) and the eluate was monitored for ENR using a fluorescence detector (Waters) at an excitation of 278 nm and an emission of 455 nm.
ENR conjugates were synthesized by linkage of the carboxylic acid group of ENR with the amino group of the carrier protein. This reaction resulted in an amide bond between the ENR and the carrier protein. To determine the effectiveness of the conjugation reaction, UV absorbances were measured for BSA, ENR, and ENR-BSA, respectively. The absorbance of ENR-BSA (271, 322 nm) shows a band at 271 nm indicating a blue shift of 3 nm compared to the absorption at 274 nm for ENR (274, 324, 330 nm). The immunogen ENR-KLH gave a UV pattern similar to that of ENR-BSA.
Fig. 1 shows the standard inhibition curve for ENR obtained using the developed ELISA system. The lowest detectable level of ENR in this ELISA was 0.7 ng/mL. To determine the specificity of the prepared antibody, cross-reactivity towards various FQs was carried out by direct competitive ELISA using ENR-BSA as the coating antigen. Cross-reactivity was measured by comparison of the IC50 to that of ENR. The IC50 values of the mAb for enrofloxacin, ciprofloxacin, difloxacin, sarafloxacin, pefloxacinand norfloxacinwere 5.0, 8.3, 9.7, 21.7, 36.0, and 63.7 ng/mL, respectively. Cross-reactivity levels of the mAb to ciprofloxacin, difloxacin, sarafloxacin, pefloxacin and norfloxacin were 60, 52, 23, 14 and 8%, respectively (Table 1).
To validatethe developed ELISA system for quantitative determination of FQs, three different matrices (chicken muscle, beef, and egg) were selected. For each matrix, known amounts of ENR were spiked at a range of 10, 20, and 30 ng/mL, then analyzed using the optimized ELISA procedure. Recoveries for ENR were observed in the range of 72.9 to 113.16%. The coefficient of variation (CV) ranged from 2.42% to 10.11% (Table 2).
A total of 130 µg of mAbs against ENR were mixed with three different amounts of MNP (1, 2, and 3 mg) in PBS to determine thesuitableamount of MNPs for mAbs coupling. Increasing the amount of MNP increased the binding efficiency to 39.49% (1 mg), 74.88% (2 mg), and 93.5% (3 mg) per 130 µg of mAb (Fig. 2). Therefore, 130 µg of mAb and 3 mg MNP were selected for further studies.
The applicability of the novel mAb-MNP system was verified by testing the recoveries for the ENR residue in three different matrices. Recoveries for ENR ranged from 75.16 to 86.36%. The coefficient of variation (CV) ranged from 5.08% to 11.53% (Table 3). The chromatogram showed that mAb-MNP conjugates could remove many matrix components from animal meat (Fig. 3).
As a light molecule with a molecular mass of 359.4, enrofloxacin must be conjugated with a carrier protein to elicit the immune response, which an animal uses to produce the desirable antibodies. Szurodoki et al. indicated that,among the carrier proteins for immunogens, KLH is generally a potent immunogen [28]. KLH conjugate was also found to much more accurately stimulate the corresponding region of the protein and to induce the synthesis of larger amounts of specific antibody than a BSA conjugate [712]. Therefore, we prepared to conjugate ENR with KLH for an immunogen and ENR with BSA for a coating antigen using the NHS method [152229].
Based on the ENR-BSA coated ELISA system, the IC50 measurements were validated by direct competitive ELISA under optimized conditions with three replicates. The IC50 data of anti-ENR-HRP conjugate binding to six FQs analogs were analyzed to investigate the relationship of the anti-ENR-HRP conjugate affinity with the structural factor of the FQ analogs. The IC50 values of the FQ analogs demonstrate that loss of the cyclopropyl group and 4-ehtyl-piperazinyl ring of ENR have various effects on antibody binding. The structures of ENR and ciprofloxacin are very similar in all respects except for the ethyl group in the piperazinyl ring, which is substituted by hydrogen. Similarly, an analogous comparison of ciprofloxacin to norfloxacin results from alkyl substitution on the piperazinyl ring and cyclopropyl group replaced by a fluorophenyl group or ethyl group, which produces an increase in the IC50 value. These results indicate the possibility that anti-ENR strongly recognizesthe cyclopropyl group and the ethyl group in the piperazinyl ring, leading to its higher reactivity with the antibody. According to other reports, the cross-reactivity of anti-ENR mAb produced in the laboratory for ciprofloxacin and sarafloxacin was100% and 16%, respectively, indicating that the antibody strongly recognized oxygen and fluorine atoms [16].
To validate the efficiency of the anti-ENR, ENR concentrations were detected by the ENR-BSA coated ELISA system when three different foods were exposed to the ENR. These results demonstratedthat these antibodies can be used in multiresidue monitoring of FQs in food samples. Based on the CODEX Alimentarius guideline (CAC/GL 71-2009) for quantitative analytical methods, acceptable CVs for intra-laboratory testing are < 15% and acceptable recoveries are 70 to 120% for samples containing 10 to 100 ng/mL analyte. The present data represents good repeatability of the developed ELISA. Kato et al. reported that indirect competitive ELISA was more sensitive than direct competitive ELISA. However, our data showed that direct competitive ELISA had excellent sensitivity, which could be affected by the strength of interaction between the ENR antibody-HRP conjugate and coating antigen [16].
In this study, we first presented a simple purification system using MNPs for fast and efficient purification of ENR and ciprofloxacin from animal meat. A covalent anti-ENR mAb binding to the MNP surface was developed based on the reaction with the amino group of the antibody molecule in an MNP concentration-dependent manner [10]. The recoveries in our study ranged from 75.16 to 86.36%, while the CV values ranged from 5.08 to 11.53%, which could sufficiently satisfy the CODEX Alimentarius guideline. The results presented herein demonstrated that many matrix components in food could be removed by using mAb-MNP conjugates to purify FQs from animal meat. We suggest that this new system can be used as a simple and effective method for purification of fluroquinolones from food matrix.
In conclusion, we have prepared a high-quality anti-ENR mAb with a high affinity for ENR and some other FQs and developed a competitive ELISA method to detect ENR and ciprofloxacin residues in three different animal meats using anti-ENR mAb. We also developed a new ENR purification tool to extract ENR from three different animal meats using mAb-MNP conjugate. The developed screening and purification method could be a useful tool for the surveillance and purification of FQ residues from livestock products.
Figures and Tables
Fig. 1
Standard curve of direct competitive enzyme-linked immunosorbent assay (ELISA) using coating antigen enrofloxacin (ENR)-bovine serum albumin (BSA) (100 ng/mL) and ENR monoclonal antibody (mAb)-HRP (diluted 1/1,000, final dilution in the well). (A) Standard curves of chicken muscle samples. (B) Standard curves of egg samples. (C) Standard curves of cattle samples. logC, ENR standard concentration in extract solution (40, 20, 10, 5, 2.5, and 0 ng/mL).

Fig. 2
Binding efficiency of ENR mAb to different amounts of magnetic nanoparticles (MNPs). ENR mAb (130 µg) was coupled with each amount of MNP (n = 3). Data shown represent the mean ± SE (n = 3).

Fig. 3
High-performance liquid chromatography chromatogram of ENR. (A) Standard sample: MeOH in distilled water (DW) containing 25 ng/mL ENR. (B) Sample: chicken muscle spiked with 25 ng/mL ENR (dilution). (C) MeOH in DW sample.

Table 1
IC50 values and cross-reactivity of anti-ENR toward selected fluoroquinolones using the optimized ELISA format
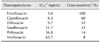
*IC50was calculated as the concentration of a competitor that caused a 50% reduction in binding of the antibody to the coating antigen. The data represent three separate experiments run in three different days. †The percentage of cross-reactivity was determined by the ratio of the test compounds IC50 to that of enrofloxacin.
Acknowledgments
This work was supported by research funds from the Animal and Plant Quarantine Agency, Korea.
References
1. Appelbaum PC, Hunter PA. The fluoroquinolone antibacterials: past, present and future perspectives. Int J Antimicrob Agents. 2000; 16:5–15.

2. Ashwin H, Stead S, Caldow M, Sharman M, Stark J, De Rijk A, Keely BJ. A rapid microbial inhibition-based screening strategy for fluoroquinolone and quinolone residues in foods of animal origin. Anal Chim Acta. 2009; 637:241–246.

3. Bucknall S, Silverlight J, Coldham N, Thorne L, Jackman R. Antibodies to the quinolones and fluoroquinolones for the development of generic and specific immunoassays for detection of these residues in animal products. Food Addit Contam. 2003; 20:221–228.

4. Cervino C, Weber E, Knopp D, Niessner R. Comparison of hybridoma screening methods for the efficient detection of high-affinity hapten-specific monoclonal antibodies. J Immunol Methods. 2008; 329:184–193.

5. Cinquina AL, Roberti P, Giannetti L, Longo F, Draisci R, Fagiolo A, Brizioli NR. Determination of enrofloxacin and its metabolite ciprofloxacin in goat milk by high-performance liquid chromatography with diode-array detection. Optimization and validation. J Chromatogr A. 2003; 987:221–226.

6. Community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin. Council Regulation (EEC). No. 2377/90. OJ. L. 1990. p. 224.
7. Daniel C, Lacroix M, Talbot PJ. Mapping of linear antigenic sites on the S glycoprotein of a neurotropic murine coronavirus with synthetic peptides: a combination of nine prediction algorithms fails to identify relevant epitopes and peptide immunogenicity is drastically influenced by the nature of the protein carrier. Virology. 1994; 202:540–549.

8. El-Boubbou K, Gruden C, Huang X. Magnetic glyconanoparticles: a unique tool for rapid pathogen detection, decontamination, and strain differentiation. J Am Chem Soc. 2007; 129:13392–13393.

9. Fan GY, Yang RS, Jiang JQ, Chang XY, Chen JJ, Qi YH, Wu SX, Yang XF. Development of a class-specific polyclonal antibody-based indirect competitive ELISA for detecting fluoroquinolone residues in milk. J Zhejiang Univ Sci B. 2012; 13:545–554.

10. Grüttner C, Müller K, Teller J, Westphal F, Foreman A, Ivkov R. Synthesis and antibody conjugation of magnetic nanoparticles with improved specific power absorption rates for alternating magnetic field cancer therapy. J Magn Magn Mater. 2007; 311:181–186.

11. Hadjipanayis CG, Machaidze R, Kaluzova M, Wang L, Schuette AJ, Chen H, Wu X, Mao H. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010; 70:6303–6312.

12. Herscowitz HB, Harold WW, Stavitsky AB. Immunochemical and immunogenic properties of a purified keyhole limpet haemocyanin. Immunology. 1972; 22:51–61.
13. Huang B, Yin Y, Lu L, Ding H, Wang L, Yu T, Zhu JJ, Zheng XD, Zhang YZ. Preparation of high-affinity rabbit monoclonal antibodies for ciprofloxacin and development of an indirect competitive ELISA for residues in milk. J Zhejiang Univ Sci B. 2010; 11:812–818.

14. Huet AC, Charlier C, Tittlemier SA, Singh G, Benrejeb S, Delahaut P. Simultaneous determination of (fluoro)quinolone antibiotics in kidney, marine products, eggs, and muscle by enzyme-linked immunosorbent assay (ELISA). J Agric Food Chem. 2006; 54:2822–2827.

15. Karu AE, Goodrow MH, Schmidt DJ, Hammock BD, Bigelow MW. Synthesis of haptens and derivation of monoclonal antibodies for immunoassay of the phenylurea herbicide diuron. J Agric Food Chem. 1994; 42:301–309.

16. Kato M, Ihara Y, Nakata E, Miyazawa M, Sasaki M, Kodaira T, Nakazawa H. Development of enrofloxacin ELISA using a monoclonal antibody tolerating an organic solvent with broad cross-reactivity to other newquinolones. Food Agric Immunol. 2007; 18:179–187.

17. Khaydarov RA, Khaydarov RR, Gapurova O. Water purification from metal ions using carbon nanoparticleconjugated polymer nanocomposites. Water Res. 2010; 44:1927–1933.

18. Lee HM, Song SO, Cha SH, Wee SB, Bischoff K, Park SW, Son SW, Kang HG, Cho MH. Development of a monoclonal antibody against deoxynivalenol for magnetic nanoparticle based extraction and an enzyme-linked immunosorbent assay. J Vet Sci. 2013; 14:143–150.

19. Liu WT. Nanoparticles and their biological and environmental applications. J Biosci Bioeng. 2006; 102:1–7.

20. Liu YZ, Zhao GX, Wang P, Liu J, Zhang HC, Wang JP. Production of the broad specific monoclonal antibody against sarafloxacin for rapid immunoscreening of 12 fluoroquinolones in meat. J Environ Sci Health B. 2013; 48:139–146.

21. Liu Z, Lu S, Zhao C, Ding K, Cao Z, Zhan J, Ma C, Liu J, Xi R. Preparation of anti-danofloxacin antibody and development of an indirect competitive enzyme? linked immunosorbent assay for detection of danofloxacin residue in chicken liver. J Sci Food Agric. 2009; 89:1115–1121.

22. Lu S, Zhang Y, Liu J, Zhao C, Liu W, Xi R. Preparation of anti-pefloxacin antibody and development of an indirect competitive enzyme-linked immunosorbent assay for detection of pefloxacin residue in chicken liver. J Agric Food Chem. 2006; 54:6995–7000.

23. Martinez JL. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ Pollut. 2009; 157:2893–2902.

24. McBain SC, Yiu HH, Dobson J. Magnetic nanoparticles for gene and drug delivery. Int J Nanomedicine. 2008; 3:169–180.
25. Mori K, Maru C, Takasuna K, Furuhama K. Mechanism of histamine release induced by levofloxacin, a fluoroquinolone antibacterial agent. Eur J Pharmacol. 2000; 394:51–55.

26. Ravindranath SP, Mauer LJ, Deb-Roy C, Irudayaraj J. Biofunctionalized magnetic nanoparticle integrated midinfrared pathogen sensor for food matrixes. Anal Chem. 2009; 81:2840–2846.

27. Spieker-Polet H, Sethupathi P, Yam PC, Knight KL. Rabbit monoclonal antibodies: generating a fusion partner to produce rabbit-rabbit hybridomas. Proc Natl Acad Sci U S A. 1995; 92:9348–9352.

28. Kurtz DA, Skerritt JH, Stanker LH. New Frontiers in Agrochemical Immunoassay. Rockville: AOAC International;1995. p. 39–63.
29. Watanabe E, Eun H, Baba K, Arao T, Endo S, Ueji M, Ishii Y. Synthesis of haptens for development of antibodies to alkylphenols and evaluation and optimization of a selected antibody for ELISA development. J Agric Food Chem. 2005; 53:7395–7403.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download