Abstract
Figures and Tables
 | Fig. 1Examples of radial velocity and strain (A-D) assessment on right parasternal short-axis view at the level of papillary muscles and longitudinal velocity and strain assessment (E-H) of left ventricle (LV) on left apical four chamber view in a healthy beagle dog. (A and B) For radial velocity, 2 × 2 mm samples were placed on the endocardial (yellow circle) and epicardial (pink circle) segments between the papillary muscles. Peak velocities in systole (Sm), early diastole (Em) and late diastole (Am) were measured in the endocardium (yellow curve) and epicardium (pink curve). (C and D) Strain and strain rate variables were determined for a 3.5 × 3.5 mm region of interest and 1.5 mm strain length on the same frame. Maximum positive systolic strain was obtained in the end-systole. (E-G) The basal (yellow circle) and apical (pink circle) segments of the left ventricular free wall and interventricular septum with a 2 × 2 mm region of interest were used in the velocity analyses. (H-J) Peak velocities in systole (Sm), early diastole (Em) and late diastole (Am) were measured. Using the same region of interest with 5 mm strain length, strain and systolic strain rate were determined on the same frame. Negative systolic strain reflects longitudinal compression. |
Table 1
Size and position of region of interest (ROI) for tissue Doppler imaging-derived parameters in left ventricle (LV)

Table 2
Within-day and between-day variabilities of tissue Doppler imaging-derived variables for both radial and longitudinal motion of the left ventricle free wall and interventricular septum in six beagles

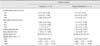
aCV < 15%. SD, standard deviation; CV, coefficient of variation; Sm, peak velocities of left ventricular myocardium at systole; Em, peak velocities of left ventricular myocardium at early diastole; Am, peak velocities of left ventricular myocardium at late diastole; SRS, systolic strain rate; SRE, early diastolic strain rate; SRA, late diastolic strain rate.
Table 3
Median values and ranges of clinical parameters in the normal and iatrogenic hypercortisolism groups

Table 4
Conventional echocardiographic parameters related to left ventricle in normal and iatrogenic hypercortisolism groups
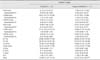
*Indicates significantly different at p < 0.05. IVSd, interventricular septal diastolic thickness; LVIDd, left ventricular end-diastolic diameter; LVFWd, left ventricular free wall diastolic thickness; IVSs, interventricular septal systolic thickness; LVIDs, left ventricular end-systolic diameter; LVFWs, left ventricular free wall systolic thickness; EF, ejection fraction; FS, fractional shortening; LA, left atrium; LA/Ao, left atrial to aortic diameter ratio; E, peak early left ventricular inflow velocity; A, peak late left ventricular inflow velocity; E/A, ratio of peak early to peak late left ventricular inflow velocities; E/Em, ratio of peak early left ventricular inflow velocities to peak radial left ventricular free wall velocity in early diastole.
Table 5
Tissue Doppler imaging-derived variables of radial left ventricular free wall in the normal and iatrogenic hypercortisolism groups




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download