Abstract
Although silver is known to be a broad-spectrum biocidal agent, the effects of this metal against Sacbrood virus have not yet been investigated. In this study, we evaluated the efficacy of silver ions against natural Korean sacbrood virus (KSBV) infection of Apis (A.) cerana. Ten KSBV-infected colonies containing A. cerana with similar strength and activity were selected from an apiary located in Bosung-gun (Korea). Among these, five colonies were randomly assigned to the treatment group that was fed sugar syrup containing 0.2 mg/L silver ions. The other colonies were assigned to the untreated control group in which bees were given syrup without the silver ions. To assess the efficacy of the silver ions, colony strength, colony activity, and the number of dead larvae per hive were measured. During the experimental period, the test group maintained its strength and activity until day 32 while those of bees in the control group decreased sharply after day 8 to 16. Survival duration of the test group was significantly longer (40 days) than that of the control group (21 days). These results strongly indicated that silver ions are effective against KSBV infection in A. cerana.
Sacbrood virus (SBV) affects honey bees and belongs to the genus Iflavirus. SVB is a non-enveloped isometric virus that has a positive single-stranded RNA genome [21]. It infects honey bees during both the larval and adult stages. Infection results in the death of larvae while obvious signs of disease are often absent during the adult stage. Larvae infected with SBV fail to pupate and ecdysial fluid rich in virus accumulates beneath the unshed skin [3]. Infected larvae undergo a change in color from white to pale yellow and eventually die. Shortly afterwards, the body of the dead larva dries out and forms a dark brown gondola-shaped scale [36]. Adult bees infected with SBV modify their foraging behavior and their life span is reduced [15]. Cytopathological effects of the virus infection are observed in nearly all tissues of a severely infected larva [15]. SBV infects both the Western honey bee Apis (A.) mellifera and the Eastern honey bee A. cerana. The pathogenicity, however, is much stronger in the Eastern honey bee [15].
SBV infection of A. mellifera was recognized for the first time in 1964 and the causative agent was identified [6]. In A. cerana, the disease was first reported in 1976 in Thailand and the pathogen was named Thai sacbrood virus (TSBV) due to physical and serological properties that are distinct from the SBV in A. mellifera [5]. After the first occurrence in Thailand, the virus spread to Hindu Kush, Himalayas, Nepal, and India. More than 95% of A. cerana colonies were eliminated in these areas, particularly in temperate regions where the disease was the most epidemic [2225]. In China, the disease was first observed in Guangdong Province in 1972 and the causative was named Chinese sacbrood virus (CSBV) based on differences in antigenic properties compared to SBV [10]. The virus subsequently spread throughout China and other regions of Southeast Asia [927]. In Korea, a widespread outbreak of SBV disease caused a fatal collapse of the A. cerana apiary industry in 2009 [8]. The causative pathogen was identified as SBV [8] and named Korean sacbrood virus (KSBV) based on differences in nucleotide and deduced amino acid identities [7].
Since SBV has spread to all over Asia and caused fatal brood disease, efforts have been made to treat and prevent the devastating disease caused by the virus among A. cerana, but no effective results have so far been obtained. In Nepal, several beekeeping techniques to control and prevent sacbrood disease were proposed [24]. The methods included feeding bees sugar syrup containing herbal medicines, using several modified hives for early detection of the disease, and sanitizing beehives to reduce sources of re-infection. Feeding CSBV dsRNA sequences to A. cerana during the larval stages was suggested in China as a way to experimentally protect bees from subsequent CSBV infection. However, the efficacy of this procedure has not been evaluated clinically [18]. Although several other methods have been introduced in attempts to control CSBV in A. cerana including the selection of resistant bee populations, clearance of infected hives, and prevention of temperature fluctuations in the hives [18], no effective protocols are currently available.
Silver has been used to produce jewelry, ornamentation, and fine cutlery. This metal has also been exploited for centuries given its medicinal properties. For example, silver was used as a remedy for tetanus and rheumatism in the 19th century as well as colds and gonorrhea before the discovery of antibiotics in the early part of the 20th century. Presently, silver is reemerging as a viable treatment option for infections encountered in burns, open wounds, and chronic ulcers [4]. It has been used in many forms including silver ion solutions, silver nanoparticles (AgNPs), and silver salt [4]. The biocidal effects of silver ions and silver salt against as many as 12 species of bacteria including Escherichia coli are well known [28]. AgNPs were found to have both antibacterial and antiviral activities [16]. For example, Lu et al. [19] reported a high binding affinity of AgNPs to hepatitis B virus (HBV) DNA and extracellular virions with different sizes ranging from 10 to 50 nm. AgNPs were also observed to have destructive effects on the influenza virus membrane glycoprotein [20]. Furthermore, it has been suggested that AgNPs bind to a viral envelope glycoprotein in a variety of HIV-1 strains to inhibit infection [17].
The efficacy of silver as a broad-spectrum biocidal agent has been clearly demonstrated. However, the ability of silver to act against SBV infection in honey bees has not yet been investigated. We therefore evaluated the effects of silver ions against natural KSBV infection in A. cerana.
An apiary of A. cerana that was naturally infected with KSBV located in Bosung-gun (Korea) was selected as the study farm. The farm used a Japanese-style 'box pile hive' that is used by most Korean A. cerana farms. Each hive consisted of a lid on the top, a number of piled-up boxes with an internal dimension of 24 × 24 × 9 cm for each box, and a bottom floor that had an entrance for the bees and space to place the sugar diet (Fig. 1). As the A. cerana colony combs expanded downwards, a new box was added from the bottom to a maximum of 10 to 12 boxes per hive. A number of dead larvae was observed on the bottom of each hive and was considered a typical sign of SBV infection.
Four to five dead larvae collected from the bottom floor of two affected hives were processed to confirm infection with KSBV. The samples were completely homogenized in sterile PBS, and RNA was extracted from the supernatant with an RNeasy Mini Kit (Qiagen, Germany) according to the manufacturer's instructions. Total RNA was recovered in 30 mL of elution buffer and used directly for RT-PCR. Two primer pairs including SB1-2 [12] and SBV [26] were chosen to target specific sequences of the SBV polyprotein genome (Table 1). RT-PCR amplification was conducted using a OneStep RT-PCR kit (Qiagen). Reverse transcription was performed at 50℃ for 30 min. PCR was then performed in a CG1-96 Thermal Cycler (Corbett Research, Australia) under the following conditions: initial denaturation at 95℃ for 5 min followed by 40 cycles of denaturation at 95℃ for 20 sec, annealing at 55℃ (for SB 1-2) or 52℃ (SBV) for 20 sec, extension at 72℃ for 1 min, and a final extension at 72℃ for 5 min.
The amplification products were purified by a MEGA quick-spin Total Fragment DNA Purification Kit (iNtRON, Korea) and directly used for sequencing (Solgent, Korea). The nucleotide sequences were identified using the Basic Local Alignment Search Tool (BLAST) of the National Center for Biotechnology Information (NCBI, USA) [2]. Multiple nucleotide alignment was carried out with BioEdit ver. 7.0.9.0 [13] using published SBV sequences as a reference.
A silver ion solution was produced by the electrochemical method using a two-electrode setup to transform silver metal into silver ions as previously reported [14]. The anode and cathode electrodes consisted of two 99.9% pure silver metal bars 120 mm in length and 40 mm in radius, and were installed parallel to each other at a distance of 40 mm. The electric current was maintained at 30 mA of 1 L/h. All processes in this study were from Positive Charge Sterilizer (B&J, Korea). Concentration of the silver ion solution was determined by inductively coupled plasma mass spectrometry (NexION 300X ICP-MS; PerkinElmer, USA) at the Center of Research Facilities of Chonnam National University (Korea).
Among the KSBV-affected bee colonies of the apiary, we chose 10 that had similar colony strength and activity. Among these, five colonies were randomly allocated to the treatment group that was provided with brown sugar syrup (0.83 kg/L water; Samyang, Korea) containing 0.2 mg/L silver ions (B&J). The other five colonies belonged to the untreated control group that was fed 200 mL of sugar syrup alone. Day 0 was defined as the first day that the honey bees were fed the sugar syrup containing silver ions. Each colony was placed 3 to 4 m apart.
Three different sizes of Styrofoam plates were stacked in a way that a small plate (17 × 10 × 1 cm) was inserted between two larger plates (20 × 15 × 3 cm) with the upper one wrapped with a high-density polyethylene bag (CLEANWRAP, Korea) to collect dead larvae and the lower one to hold the sugar syrup (Fig. 2). All three Styrofoam plates were secured with an elastic rubber band and placed on the bottom of each hive. The Styrofoam plates with fresh sugar syrup were replaced with new ones every 2 days.
The strength of each bee colony was observed from the bottom of the hive with a mirror. The strength was recorded for five levels. We divided the inner space of each hive box into 16 compartments. Each compartment was considered to be in fine condition when the honeycombs were completely covered with a sufficient number of bees and the adult worker bees appeared healthy. When the number of compartments in fine condition was 14 or more (X ≥ 88%), a score of 4 was assigned meaning that the colony was in excellent condition. When the number of compartments in fine condition was 13-10 (88 > X ≥ 63%), 9-6 (63 > X ≥ 38%), 5-2 (38 > X ≥ 13%), and less than 2 (13% > X), scores of 3, 2, 1, and 0, respectively, were assigned.
The number of guard bees was observed and a score of 0 to 3 was given for each colony on each inspection date. The score was based on the number and vitality of the guard bees. When the number of guard bees was over 30, 29-10, 9-1, or 0, the activity was scored as 3, 2, 1, and 0, respectively.
SPSS 9.0 (SPSS, USA) was used to perform the statistical analyses. Data are expressed as the mean ± standard error (SE). Student's t-test was used to evaluate differences between the control and test groups before administration of the silver ion solution. A repeated measured ANOVA was used to assess changes in the control and test groups. A Kaplan-Meier Survival Analysis was used to test for differences in survival duration.
RT-PCR products using primer pairs SB1-2 and SBV appeared as clear electrophoretic bands with the expected sizes of 487 and 258 from the two samples of the dead larvae, indicating that the bee colonies were infected with SBV. The two amplicons from each sample were then sequenced and aligned with published SBV sequences. The nucleotide sequences amplified by the SB1-2 and SBV primers were deposited into the GenBank database (NCBI, USA) accession nos. KM538971 to KM538972 for SB1-2 primer pairs and accession nos. KM538973 to KM538974 for SBV primer pairs.
Sequence analysis demonstrated that all four RT-PCR products had more than 98.8% similarity to the KSBV sequence registered in GenBank under the accession number HQ322114. When compared to the UK reference strain (AF092924), however, the sequences amplified in this study had only 89.0 to 95.2% similarity. Additionally, all sequences in this investigation showed 94.9 to 96.9% homology to the Chinese reference strain (AF469603). In the comparison to the Nepal reference strain (AF284629), similarity ranged from 93.8 to 95.1% and ranged from 93.2 to 94.6% with the German strain (AF284625). We also found that both nucleotide sequences amplified by the SB1-2 primer pair in this study contained eight specific motifs (Fig. 3) that were reported to be specific to KSBV by Choe et al. [7]. Based on these results, the virus infecting the apiary in Bosung-gun was identified as KSBV.
The scores for mean colony strength of the control and test groups are presented in Fig. 4. No significant difference in colony strength was observed between the control and test groups (p = 0.20) on day 0. However, repeated measured ANOVA results revealed a significant difference between the control and test groups after silver ion treatment from day 1 to 40 (F = 34.03, p < 0.0001). During the early treatment stages from day 8 to day 16, the strength of each colony in the control group rapidly weakened to the degree that worker bees stopped performing their daily duties including wax production, comb construction, and hive ventilation that resulted in collapse of the combs in some colonies. On the other hand, bee colonies in the test group maintained a relatively healthy condition with a sufficient number of adult bees. In the control group, the mean score ranged from 2.0 to 2.2 until day 16 after which the score sharply decreased to 0.6 ± 0.89. That of the test group (n = 5) ranged from 2.0 to 2.4 until day 32 after which it decreased rapidly to 0.2 ± 0.45 (ANOVA; F = 3.939, p < 0.01).
In the latter stages from day 25 to 40 when the population density of the bee colonies in the control group decreased, many bees absconded their hives. On day 40, all five colonies of the control group were empty while three colonies in the test group remained in their hives. As a result, the survival duration was significantly longer (p < 0.01) for the test group (40.2 ± 1.2 days) compared to the control group (21.8 ± 4.31 days).
Changes in colony activity of the control and test groups were similar to patterns observed for the strength of the bee colony (Fig. 5). On day 0, no significant difference in colony activity was found between the control and test groups (p = 0.74). During the experimental period after the silver ion treatment, however, there was a significant difference between the two groups (ANOVA; F = 2.757, p < 0.05). The mean score for the control group decreased sharply from 2.8 ± 0.84 (n = 5) to 1.0 ± 1.22 (n = 5) after day 8 post-treatment while that of the test group remained between 2 and 3 until day 32 post-treatment.
The mean numbers of dead larvae for the test and control groups were compared (Fig. 6). The mean score for the test group significantly decreased on day 8 and remained between 2.4 ± 3.36 (n = 5) to 10.4 ± 9.37 (n = 5) for the entire experimental period (p < 0.001). Otherwise that of control group showed no significant difference for the entire experimental period (p > 0.05).
In the present study, we identified the causative agent of fatal sacbrood disease in an apiary in Bosung-gun as KSBV. Additionally, treatment of the infected bee colony with silver ions was effective against SBV. This conclusion was based on several factors including colony strength, colony activity, and survival duration. Our results strongly indicated that the silver ion solution has a therapeutic but not curative efficacy against SBV infection in A. cerana.
Efficacy of the silver ions against KSBV infection in bees was demonstrated by a significantly higher mean score for both the activity and colony strength of the test group. Furthermore, bees in the test group maintained their mean activity and strength in a stable range until day 32 while the mean activity and strength of the control group decreased sharply from day 16. These results indicated that significantly more larvae fed with sugar syrup containing the silver ions reached their brood and pupal stages.
Structure of the beehive hinders the observation of health status factors such as brood nest conditions, larval health, food storage levels, and the pressure of the queen [1]. Consequently, survival duration of each bee colony was used to assess the efficacy of the silver ions against KSBV. Significant difference in the mean survival duration between the control (21 days) and test groups (40 days) was therefore another indication of the therapeutic efficacy of the silver ions.
We did not use the number of dead larvae as one of the efficacy indexes because there were at least two variations associated with interpretation of the results. Efficacy of the silver ions could have reduced the number of dead larvae. However, decreased egg laying by the queen bee or absconding of the queen bee could also have reduced the number of dead larvae.
Although silver nanoparticles have been shown to possess antiviral activity against HIV and influenza viruses [1920], we did not determine whether silver ions had such activity against KSBV. This is because the KSBV outbreak has been so severe that almost all A. cerana colonies in Korea had been destroyed and any laboratory assay of silver ions against the virus such as cytotoxicity and antiviral activity assays has not been preceded. We decided to perform a clinical trial of silver ions that are known to have antiviral activities. We expect to be able to identify an appropriate assay system for measuring the virucidal activity of silver ions against KSBV in the near future. Whether the effects of the silver ions are due to inactivation and/or inhibition of virion particles or augmentation of the host immune system against virus replication should be determined. Additionally, introduction of silver ions into the diet of susceptible bee colonies can result in contamination of honey destined for human consumption. Side effects including the accumulation of silver in soft tissues such as skin, liver, and spleen could result in argyria and have to be considered. Studies on silver bioaccumulation along with tolerable levels in drinking water and soil have been conducted [1123]. However, such investigations in bees, as well as honey and other bee products, have not been conducted. Thus, further studies on bioaccumulation in bees and safe concentrations of silver residue in honey or other bee products should be performed.
Figures and Tables
Fig. 1
Inner structure of a traditional Japanese-style beehive. (A) Hive lid. (B) Ventilation opening. (C) Hive body. (D) Empty bottom of the hive. (E) Entrance for worker bees.
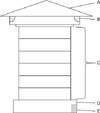
Fig. 2
A Styrofoam set consisting of three disposable plates were placed at the bottom of each beehive. Numerous dead larvae that fell from an infected beehive were observed on the top plate. Sugar syrup was placed in the bottom plate.

Fig. 3
Multiple sequence alignment of the partial SBV polyprotein gene amplified by the primer pair SB1-2 (nucleotides 241-669 according to reference sequence AF092924) comparing the Bosung-gun isolates from this study and published sequences from various geographic region. Eight motifs reported to be specific for KSBV by Choe et al. [7] are marked with rectangles.

Fig. 4
Colony strength (mean ± SE) of the test (n = 5) and control (n = 5) groups during the experimental period. Condition of the colonies was recorded at five levels. We divided the inner space of each hive box into 16 compartments. When the number of compartments in fine condition was 14 or more (X ≥ 88%), a score of 4 was assigned meaning that the colony was in excellent condition. When the number of compartments in fine condition was 13-10 (88 > X ≥ 63%), 9-6 (63 > X ≥ 38%), 5-2 (38 > X ≥ 13%), or less than 2 (13% > X), scores of 3, 2, 1, and 0, respectively, were assigned. A repeated measured ANOVA revealed a significant difference between the control and test groups after treatment with the silver ions from day 1 to 40 (F = 34.03, p < 0.0001).

Fig. 5
Colony activity (mean ± SE) of the test (n = 5) and control (n = 5) groups during the experimental period. The score was based on the number and vitality of the guard bees. When the number of guard bees was over 30, 29-10, 9-1, or 0, the activity was scored as 3, 2, 1, and 0, respectively. A repeated measured ANOVA revealed a significant difference between the control and test groups after treatment with the silver ions from day 1 to 40 (F = 2.757, p < 0.05).

Fig. 6
Number of dead larvae (mean ± SE) for the test (n = 5) and control (n = 5) groups during the experimental period. The number of dead larvae that had fallen to the bottom of each beehive and front of the entrance was counted. No significant difference between the control and test groups was observed after treatment with the silver ions according to a repeated measured ANOVA.

Acknowledgments
This study was supported by the Animal and Plant Quarantine Agency (grant no. Z-AD20-2011-11-01), Korean Ministry of Agriculture and Forestry, Korea.
References
1. Akratanakul P. Beekeeping in Asia. Rome: Food and Agricultrue Organization;1986.
2. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990; 215:403–410.

3. Anderson DL, Gibbs AJ. Transpuparial transmission of Kashmir bee virus and sacbrood virus in the honey bee (Apis mellifera). Ann Appl Biol. 1989; 114:1–7.

4. Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. Effect of silver on burn wound infection control and healing: review of the literature. Burns. 2007; 33:139–148.

5. Bailey L, Carpenter JM, Woods RD. A strain of sacbrood virus from Apis cerana. J Invertebr Pathol. 1982; 39:264–265.

6. Bailey L, Gibbs AJ, Woods RD. Sacbrood virus of the larval honey bee (Apis mellifera Linnaeus). Virology. 1964; 23:425–429.

7. Choe SE, Nguyen TTD, Hyun BH, Noh JH, Lee HS, Lee CH, Kang SW. Genetic and phylogenetic analysis of South Korean sacbrood virus isolates from infected honey bees (Apis cerana). Vet Microbiol. 2012; 157:32–40.

8. Choi YS, Lee ML, Hong IP, Kim NS, Kim HK, Lee KG, Lee ML. Occurrence of sacbrood virus in Korean apiaries from Apis cerana (Hymenoptera: Apidae). J Apic. 2010; 25:187–191.
9. Dong BY, Fang YZ, Guo ZQ, Li XJ, Zhang Y, Bi KH. Study on Chinese sacbrood bee virus. Apic China. 1986; 2:6–8.
10. Dong BY, Yuan YD, Zhang GC, Guo ZQ, Liu JS. Study on serological relationships or mutual susceptibility of CSBV and SBV. Apic China. 1984; 3:8–9.
11. Environmental Protection Agency (US). Integrated Risk Information System: Silver (CASRN 7440-22-4). Washington: US Environmental Protection Agency;1995. .
12. Grabensteiner E, Ritter W, Carter MJ, Davison S, Pechhacker H, Kolodziejek J, Boecking O, Derakhshifar I, Moosbeckhofer R, Licek E, Nowotny N. Sacbrood virus of the honeybee (Apis mellifera): rapid identification and phylogenetic analysis using reverse transcription-PCR. Clin Diagn Lab Immunol. 2001; 8:93–104.

13. Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser (Oxf). 1999; 41:95–98.
14. Jung WK, Koo HC, Kim KW, Shin S, Kim SH, Park YH. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl Environ Microbiol. 2008; 74:2171–2178.

15. Kevan PG. The Asiatic Hive Bee: Apiculture, Biology, and Role in Sustainable Development in Tropical and Subtropical Asia. Cambridge: Enviroquest;1995.
16. Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007; 3:95–101.

17. Lara HH, Garza-Treviño EN, Ixtepan-Turrent L, Singh DK. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J Nanobiotechnology. 2011; 9:30.

18. Liu X, Zhang Y, Yan X, Han R. Prevention of Chinese sacbrood virus infection in Apis cerana using RNA interference. Curr Microbiol. 2010; 61:422–428.

19. Lu L, Sun RW, Chen R, Hui CK, Ho CM, Luk JM, Lau GK, Che CM. Silver nanoparticles inhibit hepatitis B virus replication. Antivir Ther. 2008; 13:253–262.
20. Mehrbod P, Motamed N, Tabatabaian M, Soleimani Estyar R, Amini E, Shahidi M, Kheiri MT. In vitro antiviral effect of "Nanosilver" on influenza virus. Daru. 2009; 17:88–93.
21. Ongus JR, Peters D, Bonmatin JM, Bengsch E, Vlak JM, van Oers MM. Complete sequence of a picorna-like virus of the genus Iflavirus replicating in the mite Varroa destructor. J Gen Virol. 2004; 85:3747–3755.

22. Rana BS, Garg ID, Khurana SMP, Verma LR, Agrawal HO. Thai sacbrood virus of honeybees (Apis cerana indica F) in North-west Himalayas. Indian J Virol. 1986; 2:127–131.
23. Ratte HT. Bioaccumulation and toxicity of silver compounds: a review. Environ Toxicol Chem. 1999; 18:89–108.

24. Saville NM. Battling with bee brood disease in Apis cerana in W. Nepal-some findings. In : Proceeding of the Seventh IBRA Conference on Tropical Bees: Management and Diversity, and Fifth Asian Apicultural Association Conference; 19-25 March 2000; Chiang Mai, Thailand.
25. Verma LR, Rana BS, Verma S. Observations on Apis cerana colonies surviving from Thai sacbrood virus infestation. Apidologie. 1990; 21:169–174.

26. Yoo MS, Lee DW, Kim IW, Kim DS, Kwon SH, Lim YG, Yoon BS. Identification of Black queen cell virus from the honeybee in Korea. J Apic. 2007; 22:43–52.
27. Zhang J, Feng J, Liang Y, Chen D, Zhou ZH, Zhang Q, Lu X. Three-dimensional structure of the Chinese sacbrood bee virus. Sci China C Life Sci. 2001; 44:443–448.

28. Zhao G, Stevens SE Jr. Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion. Biometals. 1998; 11:27–32.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download