Abstract
Porcine circovirus type 2 (PCV2) is the primary causative agent for post-weaning, multisystemic, wasting syndrome. Consequently, serologic detection of and vaccination against PCV2 are important for the swine industry. Among several serological tests, the enzyme-linked immunosorbent assay (ELISA) is commonly used to measure anti-PCV2 antibody levels. In the present study, we used two commercial ELISA systems to comparatively evaluate anti-PCV2 antibodies in field pigs treated with three different PCV2 vaccines. Among a total of 517 serum samples, the results of the two ELISAs were fully concordant for 365 positive and 42 negative samples, indicating 78.7% agreement. In addition, the Pearson coefficient (0.636) indicated a moderate correlation between data from the two ELISAs. Results from the farms with pigs vaccinated with the three different PCV2 vaccines demonstrated that most of the vaccinated animals underwent seroconversion. However, the increase and duration of antibody titers varied depending on the vaccine, the presence of maternal antibodies, and the vaccination program. PCV2 serologic status and anti-PCV2 antibody levels of herds from this study could be utilized to determine the best timing for vaccination and assessing vaccination compliance.
Porcine circovirus (PCV; family Circoviridae, genus Circovirus) is a small non-enveloped virus with a diameter of approximately 17 nm and a circular, single-stranded DNA genome [18]. There are two types of the virus: PCV1 and PCV2. PCV2 is often associated with a number of disease symptoms in swine including enteritis, respiratory distress, systemic infection, porcine dermatitis, nephritic syndrome, and reproductive problems. These are collectively known as porcine circovirus-associated disease (PCVAD) [7,14]. In contrast, PCV1 has been shown to be nonpathogenic in pigs under experimental conditions [2,19]. Since characteristics of PCV2 that affect invasion of the immune system enhance the severity of several diseases in swine [7], PCV2-associated diseases are generally recognized as having a significant economic impact on the swine industry worldwide [8]. In addition, PCV2 interacts with other infectious or non-infectious factors (i.e., pathogens, farm type, or swine management strategies) that influence post-weaning mortality in swine [16]. Therefore, detection, surveillance, and preventive measures for PCV2 infection are important for swine producers. The virus is stable [1] and ubiquitous among swine populations [9], which makes eradication difficult. Vaccination thus represents an attractive method for controlling endemic infection and has been shown to be effective for preventing PCV2-associated diseases [5,10]. Several commercial vaccines have been developed and introduced into the swine industry.
PCV2 can be divided into two main subtypes, PCV2a and PCV2b, based on sequence analysis and composition of two open reading frames (ORFs) that encode functional proteins [3,4]. The ORF1 and ORF2 genes produce a replication-associated protein (Rep) for virus replication and an immunogenic capsid protein, respectively [3,13]. It is known that ORF2 has greater nucleotide variation than ORF1 [11]. Therefore, detection of antibodies against PCV2 differs depending on the antigens and detection methods [7,13,14].
Since several vaccines have been introduced into the Korean market, diagnostic laboratories in the country have surveyed for anti-PCV2 antibodies using different enzyme-linked immunosorbent assay (ELISA) kits. Recently, two ELISA kits with different antibody detection systems have been used in Korea. However, no studies have been performed to compare the ability to detect anti-PCV2 antibodies in field pigs resulting from PCV2 vaccines. In the current report, agreement between two commercial serologic assays using sera from field pigs vaccinated with three different PCV2 vaccines is described. Additionally, the prevalence of anti-PCV2 antibodies among pigs in Korea was evaluated.
Serum samples from pigs 20, 40, 70, 100, 130, and 160 days of age and sows were collected from farms on which the animals had been vaccinated with the three different PCV2 vaccines (Ingelvac CircoFLEX; Boehringer Ingelheim Vetmedica, USA; Circumvent PCV2 and Porcilis PCV-One; Merck Animal Health, USA). A total of 517 field pig serum samples were studied including 100, 165, and 189 samples from two, three, and three farms with pigs vaccinated with vaccine I, II, and III, respectively, as described in Table 1. The vaccinations were administered according to the recommendations of the manufacturers.
All pig sera were tested using two commercially available ELISA kits: S-ELISA (Synbiotics, France) and M-ELISA (Median Diagnostics, Korea). The ELISA protocols provided by the manufacturers were strictly followed. For the S-ELISA, the controls and serum samples (diluted 1 : 1000) were placed in wells coated with a PCV2 antigen and incubated for 1 h at 37℃. After a wash to eliminate the non-associated components, an anti-PCV2/peroxidase conjugate was added and the plates were incubated for 1 h at 37℃. When there is no specific anti-PCV2 antibody in the serum sample, the anti-PCV2/peroxidase conjugate is free to attach to the antigen in the wells. After a second wash, the enzyme conjugate was visualized by the addition of a substrate that induced a change of color. Following addition of the stop solution, the optical density was measured at 450 nm using an Emax Precision microplate reader (MDS, USA).
For the M-ELISA, the controls and serum samples (diluted 1 : 100) were incubated in wells coated with recombinant PCV2 nucleocapsid protein for 1 h at 37℃. After washing, an anti-pig immunoglobulin G (IgG)/peroxidase conjugate was added and the plates were incubated for 1 h at 37℃. Color development was induced by adding substrate solution to the plate. Optical density was measured at 405 nm using an Emax Precision microplate reader.
The S/N ratio, defined as the optical density of the test serum (S) divided by the optical density of the negative control (N), was calculated for the S-ELISA. Results for the indirect ELISA (M-ELISA) were calculated as the S/P ratio defined as the optical density of the test serum (S) divided by the optical density of the positive control (P). For the S-ELISA, samples with a ratio ≤0.4 were considered positive while ones with a ratio >0.4 were negative for antibodies. For the M-ELISA, samples with a ratio ≥0.4 were considered positive, ones with a ratio between ≥0.3 and <0.4 were false-positive, and ones with a ratio <0.3 were negative. Correlations between data from the two ELISAs were evaluated based on Pearson's correlation coefficient (r) and determination coefficients (R2) calculated with the Statistical Package for the Social Sciences software (ver. 21; IBM SPSS, USA). P values <0.05 were considered significant.
For a total of 517 serum samples, 371 (71.8%) and 472 (91.3%) had positive S-ELISA and M-ELISA results, respectively. The two ELISA assays were fully concordant for 365 positive and 42 negative (including 14 false-positive results obtained with the M-ELISA) samples, indicating 78.7% agreement for the ELISA results. There was a moderate correlation (Pearson r = -0.636, p < 0.001) between the M-ELISA results and square-root transformed S-ELISA results, indicating a linear relationship (y = -0.175x + 0.787, R2 = 0.405; Fig. 1). As shown in Fig. 1, the results for 109 samples were mismatched between the two ELISAs, including four samples with positive S-ELISA results and negative M-ELISA findings along with 105 samples with negative S-ELISA results and positive M-ELISA results. The majority of mismatches were negative for the S-ELISA and positive for the M-ELISA, which were in 0.6 ≥ S/N ratio > 0.4 of S-ELISA and 1.2 > S/P ratio ≥ 0.4 of M-ELISA.
PCV2-specific antibodies were identified in pigs vaccinated with the three different PCV2 vaccines (I, II, and III) using two commercially available ELISA kits. With vaccination, antibody titers against PCV2 were increased in most of the animals. However, the increase and duration of the antibody titer varied depending on the vaccine, presence of maternal antibodies, and vaccination program. Agreement between the ELISAs based on the vaccines was also assessed, and was 76%, 73.9%, or 83% for the pigs given the vaccine I, II, and III, respectively. In addition, results of the ELISAs had a moderate correlation (vaccine I, Pearson's r = -0.602, p < 0.001; vaccine II, r = -0.672, p < 0.001; vaccine III, r = -0.621, p < 0.001) and a linear relationship (vaccine I, y = -0.183x + 0.806, R2 = 0.362; vaccine II, y = -0.191x + 0.815, R2 = 0.451; vaccine III, y = -0.158x + 0.759, R2 = 0.386, p < 0.001) according to the vaccine (panel B in Fig. 2).
Pigs administered I vaccine had different ELISA results at 20 and 70 days of age. The S-ELISA produced negative results at 20 days of age before the antibody levels gradually increased. The M-ELISA produced positive results at 20 days of age before the levels gradually increased after a sudden decline at 70 days of age. For the pigs given vaccine II, the M-ELISA results were positive at all ages and indicated that a seroconversion occurred after the second vaccination. The S-ELISA results showed that antibody levels gradually increased, but the results for some farms (Farm 5) indicated that antibody levels decreased after vaccination. A slight decrease of antibody levels occurred at 40 days of age according to the results of both ELISAs. Pigs treated with the vaccine III showed a gradual increase in antibody levels according to both ELISAs with a significant decrease at 40 days of age based on the S-ELISA findings.
Vaccination is an attractive measure for controlling PCV2-associated diseases. Therefore, methods for measuring anti-PCV2 antibodies have been developed and applied to field samples. Serum-virus neutralization, immunoperoxidase monolayer, and indirect immunofluorescent assays have all been widely used to detect anti-PCV2 antibodies [12,17]. These methods can be labor-intensive and time-consuming, and are associated with the risk of virus contamination. In contrast, ELISAs can avoid these problems and perform large-scale diagnostics [17]. The two commercial ELISA kits used in this study involve different antigens and systems. Both ELISA kits involve PCV2 antigen; however, the S-ELISA uses a manufactured version of the antigen while the M-ELISA employs a recombinant nucleocapsid antigen of the Korean isolate. In addition, the M-ELISA is an indirect ELISA while the S-ELISA is a competitive ELISA. Therefore, the results of these two methods are interpreted differently. For example, if there is no specific anti-PCV2 antibody in the serum sample, the second antibody/conjugate of the M-ELISA does not bind to the antigen while the second antibody/conjugate of the S-ELISA is free to attach to the antigen. Using these different ELISA systems, anti-PCV2 antibody concentrations were comparatively evaluated in field pig sera in the present study.
Pearson's coefficient indicated a moderate correlation with 78.7% agreement between the ELISA results. In addition, similar levels of agreement between the ELISAs dependent upon the vaccines was observed with 76%, 73.9%, and 83% for vaccine I, II, and III, respectively. Pearson's coefficients concurred with these findings. It was therefore concluded that the ELISA results based on vaccine type were not significantly different because vaccine I, II, and III use the ORF2 of PCV2 as the antigen.
According to the results of the ELISAs for the field pigs, animals with high levels of anti-PCV2 antibodies at 20 days of age showed a slight increase or maintained antibody levels after vaccination. In addition, slight decreases in antibody levels were observed at 40 days of age in these pigs. This phenomenon was possibly due to maternal-derived antibodies (MDA) that blocked or attenuated responses to the PCV2 vaccination. MDA could interfere with development of an active humoral immune response after PCV2 vaccination [6]. However, this does not mean that vaccine efficacy is reduced because both humoral and cellular immune responses are required for protection [6,15]. In the pigs showing a low level of PCV2 antibody at 20 days of age, there was a significant seroconversion after vaccination.
The M-ELISA results had a broad positive S/P ratio ≥ 0.4 while the S-ELISA had a relatively narrow positive range of this ratio (0 ≤ S/N ratio ≤ 0.4). Therefore, the M-ELISA can be used to detect seroconversion after vaccination more easily than the S-ELISA. Although the results of the two ELISAs revealed similar seroconversion patterns in the field pigs, results for the M-ELISA were more frequently positive than those for the S-ELISA. Data from the ELISAs were controversial at some points depending on different ELISA antigens and practices at each farm. Therefore, further study of samples that produce positive M-ELISA findings and negative S-ELISA results is needed.
In summary, anti-PCV antibodies in pigs were evaluated with two commercially available ELISA kits. Both assays produced comparable results with some exceptions. Based on our findings, the levels of anti-PCV2 antibodies may be used to determine optimal timing of vaccination and verify vaccination compliance of herds.
Figures and Tables
Fig. 1
Comparison of data from the two available enzyme-linked immunosorbent assay (ELISA) kits using 517 field-collected pig sera. ELISA index values of the S-ELISA were square-root transformed. The regression equation is: y = -0.175x + 0.787; R-square = 0.405, p < 0.001.
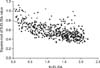
Fig. 2
Detection of PCV2-specific antibodies in field pig serum samples from animals of different ages using two commercial ELISA kits. Anti-PCV2 antibodies were verified in samples from farms with pigs vaccinated with three different PCV2 vaccines (I, II, and III) using commercially available ELISA kits (S and M). The levels of PCV2-specific antibodies are shown in a box-and-whisker plot graph displaying the minimum, first quartile, median, third quartile, and maximum. The dotted lines indicate the median level for each farm. The y-axis represents the S/N ratio and S/P ratio for the S-ELISA and M-ELISA, respectively.

Table 1
Data for pig farms that participated in the present study

PRRS: porcine reproductive and respiratory syndrome, ne: not estimated. *The three PCV2 vaccines are based on the open reading frame 2 (ORF2) protein of PCV2 and produced by different manufacturers. †A total of 517 field pig serum samples included 454 samples mentioned in this table and 63 samples from animals without a history of vaccination.
Acknowledgments
This study was supported by MSD Animal Health Korea and the Research Institute for Veterinary Science, Seoul National University, Korea.
References
1. Allan GM, Phenix KV, Todd D, McNulty MS. Some biological and physico-chemical properties of porcine circovirus. Zentralbl Veterinarmed B. 1994; 41:17–26.

2. Allan GM, McNeilly F, Cassidy JP, Reilly GA, Adair B, Ellis WA, McNulty MS. Pathogenesis of porcine circovirus; experimental infections of colostrum deprived piglets and examination of pig foetal material. Vet Microbiol. 1995; 44:49–64.

4. Cheung AK, Lager KM, Kohutyuk OI, Vincent AL, Henry SC, Baker RB, Rowland RR, Dunham AG. Detection of two porcine circovirus type 2 genotypic groups in United States swine herds. Arch Virol. 2007; 152:1035–1044.

5. Fort M, Sibila M, Allepuz A, Mateu E, Roerink F, Segalés J. Porcine circovirus type 2 (PCV2) vaccination of conventional pigs prevents viremia against PCV2 isolates of different genotypes and geographic origins. Vaccine. 2008; 26:1063–1071.

6. Fraile L, Grau-Roma L, Sarasola P, Sinovas N, Nofrarìas M, López-Jimenez R, López-Soria S, Sibila M, Segalés J. Inactivated PCV2 one shot vaccine applied in 3-week-old piglets: improvement of production parameters and interaction with maternally derived immunity. Vaccine. 2012; 30:1986–1992.

7. Ge M, Luo W, Jiang D, Li R, Zhao W, Chen G, Yang X, Yu X. Development and application of a double-antigen sandwich enzyme-linked immunosorbent assay for detection of antibodies to porcine circovirus 2. Clin Vaccine Immunol. 2012; 19:1480–1486.

8. Gillespie J, Opriessnig T, Meng XJ, Pelzer K, Buechner-Maxwell V. Porcine circovirus type 2 and porcine circovirus-associated disease. J Vet Intern Med. 2009; 23:1151–1163.

9. Harding JCS. The clinical expression and emergence of porcine circovirus 2. Vet Microbiol. 2004; 98:131–135.

10. Kixmöller M, Ritzmann M, Eddicks M, Saalmüller A, Elbers K, Fachinger V. Reduction of PMWS-associated clinical signs and co-infections by vaccination against PCV2. Vaccine. 2008; 26:3443–3451.

11. Larochelle R, Magar R, D'Allaire S. Genetic characterization and phylogenetic analysis of porcine circovirus type 2 (PCV2) strains from cases presenting various clinical conditions. Virus Res. 2002; 90:101–112.

12. Meerts P, Misinzo G, Lefebvre D, Nielsen J, Bøtner A, Kristensen CS, Nauwynck HJ. Correlation between the presence of neutralizing antibodies against porcine circovirus 2 (PCV2) and protection against replication of the virus and development of PCV2-associated disease. BMC Vet Res. 2006; 2:6.

13. Patterson AR, Johnson J, Ramamoorthy S, Meng XJ, Halbur PG, Opriessnig T. Comparison of three enzyme-linked immunosorbent assays to detect Porcine circovirus-2 (PCV-2)-specific antibodies after vaccination or inoculation of pigs with distinct PCV-1 or PCV-2 isolates. J Vet Diagn Invest. 2008; 20:744–751.

14. Patterson AR, Johnson JK, Ramamoorthy S, Hesse RA, Murtaugh MP, Puvanendiran S, Pogranichniy RM, Erickson GA, Carman S, Hause B, Meng XJ, Opriessnig T. Interlaboratory comparison of Porcine circovirus-2 indirect immunofluorescent antibody test and enzyme-linked immunosorbent assay results on experimentally infected pigs. J Vet Diagn Invest. 2011; 23:206–212.

15. Seo HW, Park C, Han K, Chae C. Effect of porcine circovirus type 2 (PCV2) vaccination on PCV2-viremic piglets after experimental PCV2 challenge. Vet Res. 2014; 45:13.

16. Serrano E, Lpez-Soria S, Trinchera L, Segals J. The use of null models and partial least squares approach path modelling (PLS-PM) for investigating risk factors influencing post-weaning mortality in indoor pig farms. Epidemiol Infect. 2014; 142:530–539.

17. Sun SQ, Guo HC, Sun DH, Yin SH, Shang YJ, Cai XP, Liu XT. Development and validation of an ELISA using a protein encoded by ORF2 antigenic domain of porcine circovirus type 2. Virol J. 2010; 7:274.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download