Abstract
Inducible cyclooxygenase-2 (COX-2) has received much attention because of its role in neuro-inflammation and synaptic plasticity. Even though COX-2 levels are high in healthy animals, the function of this factor in adult neurogenesis has not been clearly demonstrated. Therefore, we performed the present study to compare the effects of pharmacological and genetic inhibition of COX-2 on adult hippocampal neurogenesis. Physiological saline or the same volume containing celecoxib was administered perorally every day for 5 weeks using a feeding needle. Compared to the control, pharmacological and genetic inhibition of COX-2 reduced the appearance of nestin-immunoreactive neural stem cells, Ki67-positive nuclei, and doublecortin-immunoreactive neuroblasts in the dentate gyrus. In addition, a decrease in phosphorylated cAMP response element binding protein (pCREB) at Ser133 was observed. Compared to pharmacological inhibition, genetic inhibition of COX-2 resulted in significant reduction of neural stem cells, cell proliferation, and neuroblast differentiation as well as pCREB levels. These results suggest that COX-2 is part of the molecular machinery that regulates neural stem cells, cell proliferation, and neuroblast differentiation during adult hippocampal neurogenesis via pCREB. Additionally, genetic inhibition of COX-2 strongly reduced neural stem cell populations, cell proliferation, and neuroblast differentiation in the dentate gyrus compared to pharmacological inhibition.
Cyclooxygenase (COX) exists as two subtypes: COX-1 and COX-2. The participation of constitutive COX-1 in neuroinflammation has been studied, and the inhibitory role of this factor in adult neurogenesis has been recently demonstrated [33]. COX-2, which is induced in response to inflammatory stimuli, plays an essential role in the pathological process of neurodegeneration [511] and tumor formation [14]. In the central nervous system, inhibition of COX-2 specifically attenuates the deleterious effects of amyotrophic lateral sclerosis [5], Alzheimer's disease [11], infarction [4], radiation injury [17], and epilepsy [35]. Physiologically, COX-2 is expressed at lower levels under normal conditions compared to inflammatory states. Additionally, the expression of this protein in the brain and kidneys is higher than that in other organs [237]. Research on the physiological role of COX-2 has been conducted, especially in relation to synaptic plasticity, using electrophysiological techniques [340]. Furthermore, COX-2 was found to be involved in memory acquisition [29], consolidation [38], and retrieval [36] in the hippocampus. During the process of memory formation, new neurons generated by adult hippocampal neurogenesis are primarily responsible for long-term potentiation (LTP) [2021]. It has been reported that the two forms of synaptic plasticity, LTP [2021] and long-term depression (LTD) [1], are involved in the underlying mechanism of memory.
Celecoxib specifically inhibits COX-2 [10] by binding to the upper portion of the active site, thereby preventing its substrate arachidonic acid from entering the active site [7]. In COX-2 knockout (COX-2-KO) mice, the prostaglandin-endoperoxide synthase 2 (Ptgs2) gene, which encodes a rate-limiting enzyme that transforms arachidonic acid into prostaglandin H2 (PGH2) via prostaglandin G2 (PGG2), is disrupted [18]. Studies have shown that celecoxib inhibits the growth and proliferation of human neural stem cells [13] and cells in the rat dentate gyrus [12]. Genetic inhibition of COX-2 significantly decreases cell proliferation in the ischemic dentate gyrus [34]. In addition, we recently demonstrated that the genetic inhibition of COX-2 significantly reduces neurogenesis [26]. However, few studies have compared the effects of pharmacological and genetic inhibition of COX-2 on hippocampal neurogenesis. We therefore conducted the present study to compare the effects of COX-2 inhibition and deletion on adult hippocampal neurogenesis using immunohistochemistry to detect marker proteins for neural stem cells, cell proliferation, and neuronal differentiation.
Eight-week-old male COX-2-KO and wild-type mice were purchased from Taconic (USA). The COX-2-KO mice used in this study were developed at the University of North Carolina [24] and produced by Taconic. on a C57BL/6 and 129P2/Ola mixed background. The animals were from different litters and housed under specific pathogen-free conditions with adequate temperature (22℃) and humidity (60%) control as well as a 12-h light/dark cycle. All mice had free access to food and tap water. The handling and care of the mice were conduced according to guidelines that comply with current international laws and policies (National Institutes of Health [NIH] Guide for the Care and Use of Laboratory Animals, NIH publication no. 85-23, 1985, revised 1996), and were approved by the Institutional Animal Care and Use Committee of Seoul National University (approval no. SNU-120210-1), Korea. All experiments were conducted to minimize both the number of animals used and suffering due to the procedures performed.
The 9-week-old animals were divided into two groups: wild-type (n = 10), and COX-2-KO (n = 5). To compare the effects of pharmacological and genetic inhibition of COX-2 on adult neurogenesis, the wild-type animals were further divided into two subgroups (n = 5 in each group) that were treated with vehicle (physiological saline; V) or celecoxib (prescription formulation, Pfizer; COX-I). The animals were randomized for peroral administration of vehicle (1 mL/100 g body weight) or the same volume containing celecoxib (30 mg/kg body weight) in physiological saline using a feeding needle every day for 5 weeks before sacrifice. The dose of celecoxib was selected according to a previous study on the effect of COX-2 inhibition in the brain [39].
Body weight was measured on Monday of every week and at the end of the experiment. Food intake was measured and corrected for spillage by weighing the jars containing food every week between 9.00 to 10.00 h. Data are expressed as gram/day/body weight (g).
For histology, V, COX-I and COX-2-KO animals were anesthetized with 30 mg/kg Zoletil 50 (Virbac, France) with 0.1 M phosphate-buffered saline (PBS; pH 7.4) delivered via transcardial perfusion followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The brains were removed and post-fixed in the same fixative for 12 h. The brain tissues were then dehydrated with graded concentrations of alcohol before embedding in paraffin. Serial sections (3-µm thick) were cut using a microtome (Leica Biosystems, Germany) and mounted onto silane-coated slides (Muto Pure Chemicals, Japan).
To stain for COX-2, nestin, Ki67, doublecortin (DCX), and cAMP response element binding protein phosphorylated at Ser133 (pCREB), the sections were carefully processed simultaneously under identical conditions. Brain tissue sections between -1.46 and -2.46 mm to the bregma in reference to a mouse atlas [8] were selected for each animal. Antigen retrieval and immunostaining were performed as described in our previous study [26]. The sections were incubated overnight with primary antibodies specific for COX-2 (1 : 200; Cayman Chemical, USA), nestin (1 : 250; Novus Biologicals, USA), Ki67 (1 : 1,000; Abcam, UK), DCX (1 : 50; Santa Cruz Biotechnology, USA) or pCREB (1 : 1,000; Millipore, USA). Subsequently, the sections were incubated with biotinylated secondary antibodies and streptavidin peroxidase complex (Vector Labs, USA). Antibody binding was detected with 3,3'-diaminobenzidine tetrahydrochloride (Sigma, USA). The numbers of cells positive for Ki67, DCX, or pCREB in all the groups were measured. Additional, the relative optical density (ROD) of a region of interest in the dentate gyrus was measured using an image analysis system described in a previous study [26].
Body weight was not significantly different among the groups. However, the body weight and food intake of the COX-I group was increased compared to the other groups (Fig. 1).
In the V group, COX-2 immunoreactivity was detected predominantly in the cytoplasm of granule cells as well as a few interneurons in the polymorphic layer of the dentate gyrus (panel A in Fig. 2). In contrast, the COX-I and COX-2-KO groups had significantly reduced levels of COX-2 immunoreactivity in the dentate gyrus (panels B-D in Fig. 2).
For the V group, nestin-expressing neural stem cells were observed primarily in the subgranular zone of the dentate gyrus and their fibers extended to the granule cell layer (panel A in Fig. 3). In the COX-I group, nestin immunoreactivity was 80.03% of that found in the V group (panels B and D in Fig. 3). Additionally, nestin immunoreactivity observed for the COX-2-KO mice was 57.83% of that observed in the V group (panels C and D in Fig. 3).
In the V mice, Ki67-immunoreactive nuclei were clustered in the subgranular zone of the dentate gyrus (panel A in Fig. 4). The average number of Ki67-positive nuclei was 8.57. For the COX-I group, the average number of Ki67-positive nuclei was moderately reduced (6.00) compared to that in the V group (panels B and D in Fig. 4). The average number of Ki67-positive nuclei in the COX-2-KO mice (4.14) was the lowest among all groups (panels C and D in Fig. 4).
In the V group, DCX-immunoreactive neuroblasts in the subgranular zone of the dentate gyrus had a round cytoplasm, and some of the cells had well-developed dendrites (panels A and B in Fig. 5). The average number of DCX-positive neuroblasts was 24.43 per section (panel G in Fig. 5). For the COX-I group, the average number of DCX-positive neuroblasts in the dentate gyrus decreased to 16.01 per section (panels C and G in Fig. 5). However, DCX-immunoreactive dendrites were also well developed in the COX-I group compared to the V mice (panel D in Fig. 5). DCX immunoreactivity decreased to 75.06% of that found in the V group (panel H in Fig. 5). In the COX-2-KO animals, the average number of DCX-positive neuroblasts per section in the subgranular zone was the lowest (8.86), and the dendrites were poorly developed in the dentate gyrus (panels E, F, and G in Fig. 5). DCX-specific immunoreactivity in the COX-2-KO group was also the weakest (48.53% of that in the V mice) among all groups (panel H in Fig. 5).
In the V group, pCREB-positive nuclei were mainly detected in the subgranular zone of the dentate gyrus. The average number of pCREB-immunoreactive nuclei was 13.6 per section (panels A and D in Fig. 6). For the COX-I animals, the average number of pCREB-positive nuclei decreased to 10.2 per section (panels B and D in Fig. 6). In the COX-2-KO group, the number of pCREB-positive nuclei decreased significantly (5.6 per section) compared to the V and COX-I groups (panels C and D in Fig. 6).
In our present study, we observed the effects of COX-2 pharmacological and genetic inhibition on neural stem cells, cell proliferation, and neuroblast differentiation in the dentate gyrus. First, the administration of celecoxib or genetic COX-2 inhibition significantly reduced COX-2 immunoreactivity in the hippocampal dentate gyrus. Decreased COX-2 immunoreactivity was prominent in the COX-2-KO group. These results suggest that the pharmacological and genetic methods efficiently reduce COX-2 expression in the hippocampal dentate gyrus.
Next, we observed decreased neural stem cell numbers, cell proliferation, and neuronal differentiation in the dentate gyrus after pharmacological and genetic inhibition of COX-2. The reduction was more obvious in the COX-2-KO group than in the COX-I group. These results indicate that the physiological role of COX-2 in the hippocampal dentate gyrus is closely related to adult neurogenesis. In addition, decreased nestin immunoreactivity in the COX-2-KO group suggests that COX-2 knockout affected the neural stem cell pool whereas celecoxib treatment did not cause significant reduction of nestin immunoreactivity. There are several reasons for the varying degree of reduction between genetic and pharmacological inhibition. First, the celecoxib metabolite is excreted in urine and feces [27]. Therefore, the inhibition of COX-2 is not consistent like it is with systemic knockout. Yamamoto et al. [39] reported that administrating celecoxib twice per day is more effective than a single treatment. Thus, the inhibition of COX-2 can taper off until the next round of drug administration. Second, the point of initiation for the knockout condition and pharmacological inhibition condition was different. Reduction of COX-2 by knockout begins during development whereas suppression of COX-2 begins on the first day of drug administration. COX-2 KO affects the neural stem cell pool because COX-2 plays a role in postnatal brain development. COX-2 expression peaks 3 to 4 weeks following birth, after which the levels decrease [16]. Third, reduced neural stem cell pools resulted in decreased cell proliferation and neuronal differentiation in the knockout group whereas reduction of neural stem cell populations by celecoxib was not significant. Knockout effects are first observed during the development stage. Neural stem cells populate the subgranular zone during the postnatal period and persist throughout the lifetime [32]. Celecoxib may suppress COX-2 action during the conversion of neural stem cells into early post-mitotic neuroblasts. Based on these findings, we suggest that the genetic inhibition of COX-2 is more effective than treatment with inhibitors to observe the role of this factor.
Other studies including our previous work have demonstrated a correlation between COX-2 and adult hippocampal neurogenesis [9122634]. However, neurogenesis was consistently observed in the COX-2-KO group although we observed that COX-2 knockout affected neural stem cell numbers, cell proliferation, and neuronal differentiation. Based on these results, we suggest that the expression of COX-2 is important for adult neurogenesis. The molecules involved in maintaining neurogenesis are presently unknown, and further studies are required to reveal their identities.
Unlike the specific COX-2 inhibitor used in our study, treatment with a non-selective COX inhibitor does not affect hippocampal neurogenesis compared to the control groups [22]. We believe that these inconsistent results can be explained by the use of female rats and lower doses of the inhibitor in the previous study compared to the current investigation. Moreover, a non-selective COX inhibitor can suppress both neurogenic-inhibitory COX-1 and neurogenic-required COX-2 pathways [123334]. On the other hand, studies using COX-2 selective inhibitors, which exclusively suppressed COX-2 expression, produced results similar to ours [9]. In addition, celecoxib can negatively affect adult hippocampal neurogenesis by inhibiting angiogenesis and thereby cause a subsequent decrease in endothelial angiogenic stimulation [615].
We also hypothesized that pCREB, the active form of CREB, may be an important factor for COX-2-mediated neurogenesis. CREB phosphorylation is dependent on neuronal activity and is functionally linked to memory formation [1423]. In the COX-I group, there were fewer pCREB-positive nuclei, and the degree of reduced CREB phosphorylation was significant in the COX-2-KO group. These results suggest that the pattern of CREB phosphorylation is related to COX-2 expression. In addition, our present study and other studies have demonstrated the association of COX-2 and pCREB with adult neurogenesis [12252834]. Even though the interrelation between COX-2 and pCREB is unclear, the COX-2 gene has been reported as a target of pCREB [19]. Reduced levels of pCREB in the COX-I and COX-2-KO groups suggest that COX-2 may function as an upstream factor or regulator of pCREB. A possible pathway could involve increased levels of cAMP mediated by the COX-2 end product prostaglandin E2 (PGE2) [30] and subsequent activation of CREB by phosphorylation in a cAMP-dependent manner [31].
In conclusion, our study demonstrated that disruption of COX-2 expression affects neural stem cell numbers, cell proliferation, and neuroblast differentiation via the phosphorylation of CREB. In addition, the genetic inhibition of COX-2 is more efficient than its pharmacological inhibition and it is associated with an accumulating effect from development stage.
Figures and Tables
 | Fig. 1Changes in body weight (A) and food intake (B) of vehicle (V), celecoxib-treated (COX-I), and cyclooxygenase-2 (COX-2) knockout (COX-2-KO) mice (n = 5 per group). |
 | Fig. 2COX-2 immunoreactivity in the dentate gyrus of the V (A), COX-I (B), and COX-2-KO (C) groups. In the V animals, COX-2 immunoreactivity (arrows) was observed in the granule cell layer (GCL) and polymorphic layer (PL) of the dentate gyrus. In the COX-I and COX-2-KO groups, COX-2 immunoreactivity was weak compared to that in the V group. (D) The relative optical density (ROD) of the V, COX-I, and COX-2-KO groups (n = 5 per group) expressed as a percentage of the value for COX-2 immunoreactivity in the dentate gyrus of the V group per section; *p < 0.05, indicating a significant difference relative to the V group. All data are presented as the mean values ± standard error of the mean (SEM). ML: molecular layer, GCL: granule cell layer, PoL: polymorphic layer. Scale bar = 25 µm. |
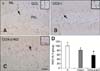 | Fig. 3Immunohistochemical staining for nestin expression in the dentate gyrus of the V (A), COX-I (B), and COX-2-KO (C) groups. Nestin-positive cells and fibers were observed in the granular cell layer (GCL) and subgranular zone of the dentate gyrus. Note that nestin-expressing cells and fibers were weakly detected in the COX-I and COX-2-KO groups compared to the V group. The degree of nestin reduction was prominent in the COX-2-KO mice compared to the COX-I group. (D) The ROD expressed as a percentage of the value for nestin immunoreactivity in the dentate gyrus per section of the V, COX-I, and COX-2-KO groups (n = 5 per group; *p < 0.05, indicating a significant difference relative to V group). All data are presented as the mean ± SEM. Scale bar = 50 µm. |
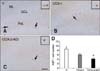 | Fig. 4Immunohistochemical staining for Ki67 in the dentate gyrus of the V (A), COX-I (B), and COX-2-KO (C) groups. Ki67-positive nuclei were observed in the subgranular zone (arrows) of the dentate gyrus. Few Ki67-positive nuclei were found in the COX-2-KO group compared to the V and COX-I groups. In particular, the number of Ki67-positive nuclei was noticeably decreased in the COX-2-KO group compared to the other groups. (D) The mean number of Ki67-positive nuclei per section in all groups (n = 5 per group; *p < 0.05, indicating a significant difference relative to the V group). All data are shown as the mean ± SEM. Scale bar = 50 µm. |
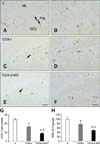 | Fig. 5Immunohistochemical staining for DCX in the dentate gyrus of the V (A and B), COX-I (C and D), and COX-2-KO (E and F) groups. DCX-immunoreactive neuroblasts were detected in the subgranular zone (arrows) of the dentate gyrus. Note that the number of cell bodies and dendrites of DCX-positive neuroblasts were prominently decreased in the COX-2-KO group compared to the V and COX-I groups. (G and H) The mean number of DCX-immunoreactive neuroblasts per section (G) and ROD expressed as a percentage of DCX immunoreactivity per section (H) in the dentate gyrus of the V, COX-I, and COX-2-KO groups relative to the V group (n = 5 per group; ap < 0.05, indicating a significant difference compared to the V group; bp < 0.05, a significant difference relative to the COX-I group). All data are presented as the mean ± SEM. Scale bars = 50 µm (A, C, and E) or 25 µm (B, D, and F). |
 | Fig. 6Immunohistochemical staining for cAMP response element binding protein phosphorylated at Ser133 (pCREB) in the dentate gyrus of the V (A), COX-I (B), and COX-2-KO (C) groups. Note that the number of pCREB-positive nuclei (arrows) decreased in both the COX-I and COX-2-KO groups compared to the V group. The number of pCREB-immunoreactive nuclei in the COX-2-KO group was more noticeably decreased compared to the COX-I groups. (D) The mean number of pCREB-positive nuclei per section in all groups (n = 5 per group; ap < 0.05, indicating a significant difference compared to the V group; bp < 0.05, a significant difference relative to the COX-I group). All data are presented as the mean ± SEM. Scale bar = 50 µm. |
Acknowledgments
This research was supported by Korea Mouse Phenotyping Project (2013M3A9D5072550) of the Ministry of Science, ICT and Future Planning through the National Research Foundation (NRF), Korea.
References
1. Aasebø IEJ, Blankvoort S, Tashiro A. Critical maturational period of new neurons in adult dentate gyrus for their involvement in memory formation. Eur J Neurosci. 2011; 33:1094–1100.

2. Breder CD, Dewitt D, Kraig RP. Characterization of inducible cyclooxygenase in rat brain. J Comp Neurol. 1995; 355:296–315.

3. Chen C, Magee JC, Bazan NG. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J Neurophysiol. 2002; 87:2851–2857.

4. Doré S, Otsuka T, Mito T, Sugo N, Hand T, Wu L, Hurn PD, Traystman RJ, Andreasson K. Neuronal overexpression of cyclooxygenase-2 increases cerebral infarction. Ann Neurol. 2003; 54:155–162.

5. Drachman DB, Frank K, Dykes-Hoberg M, Teismann P, Almer G, Przedborski S, Rothstein JD. Cyclooygenase 2 inhibition protects motor neurons and prolongs survival in a transgenic mouse model of ALS. Ann Neurol. 2002; 52:771–778.

6. Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003; 18:2803–2812.

7. FitzGerald GA, Loll P. COX in a crystal ball: current status and future promise of prostaglandin research. J Clin Invest. 2001; 107:1335–1337.
8. Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Figure 43-51. San Diego: Academic Press;1997.
9. Goncalves MB, Williams EJ, Yip P, Yáñez-Muñoz RJ, Williams G, Doherty P. The COX-2 inhibitors, meloxicam and nimesulide, suppress neurogenesis in the adult mouse brain. Br J Pharmacol. 2010; 159:1118–1125.

11. Hoozemans JJM, Rozemuller AJM, Janssen I, De Groot CJA, Veerhuis R, Eikelenboom P. Cyclooxygenase expression in microglia and neurons in Alzheimer's disease and control brain. Acta Neuropathol. 2001; 101:2–8.

12. Hwang IK, Yi SS, Yoo KY, Park OK, Yan B, Kim IY, Kim YN, Song W, Moon SM, Won MH, Seong JK, Yoon YS. Effects of treadmill exercise on cyclooxygenase-2 in the hippocampus in type 2 diabetic rats: correlation with the neuroblasts. Brain Res. 2010; 1341:84–92.

13. Jung KH, Chu K, Lee ST, Kim J, Sinn DI, Kim JM, Park DK, Lee JJ, Kim SU, Kim M, Lee SK, Roh JK. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol Dis. 2006; 23:237–246.

14. Kaltschmidt B, Ndiaye D, Korte M, Pothion S, Arbibe L, Prüllage M, Pfeiffer J, Lindecke A, Staiger V, Israël A, Kaltschmidt C, Mémet S. NF-κB regulates spatial memory formation and synaptic plasticity through protein kinase A/CREB signaling. Mol Cell Biol. 2006; 26:2936–2946.

15. Kang KB, Wang TT, Woon CT, Cheah ES, Moore XL, Zhu C, Wong MC. Enhancement of glioblastoma radioresponse by a selective COX-2 inhibitor celecoxib: inhibition of tumor angiogenesis with extensive tumor necrosis. Int J Radiat Oncol Biol Phys. 2007; 67:888–896.

16. Kaufmann WE, Worley PF, Taylor CV, Bremer M, Isakson PC. Cyclooxygenase-2 expression during rat neocortical development and in Rett syndrome. Brain Dev. 1997; 19:25–34.

17. Kyrkanides S, Moore AH, Olschowka JA, Daeschner JC, Williams JP, Hansen JT, O'Banion MK. Cyclooxygenase-2 modulates brain inflammation-related gene expression in central nervous system radiation injury. Brain Res Mol Brain Res. 2002; 104:159–169.

18. Langenbach R, Loftin C, Lee C, Tiano H. Cyclooxygenase knockout mice: models for elucidating isoform-specific functions. Biochem Pharmacol. 1999; 58:1237–1246.
19. Lee B, Dziema H, Lee KH, Choi YS, Obrietan K. CRE-mediated transcription and COX-2 expression in the pilocarpine model of status epilepticus. Neurobiol Dis. 2007; 25:80–91.

20. Malleret G, Alarcon JM, Martel G, Takizawa S, Vronskaya S, Yin D, Chen IZ, Kandel ER, Shumyatsky GP. Bidirectional regulation of hippocampal long-term synaptic plasticity and its influence on opposing forms of memory. J Neurosci. 2010; 30:3813–3825.

21. Miyamoto E. Molecular mechanism of neuronal plasticity: induction and maintenance of long-term potentiation in the hippocampus. J Pharmacol Sci. 2006; 100:433–442.

22. Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003; 302:1760–1765.

23. Moore AN, Waxham MN, Dash PK. Neuronal activity increases the phosphorylation of the transcription factor cAMP response element-binding protein (CREB) in rat hippocampus and cortex. J Biol Chem. 1996; 271:14214–14220.

24. Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, Mahler JF, Kluckman KD, Ledford A, Lee CA, Smithies O. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995; 83:473–482.

25. Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, Zhang YJ, Nestler EJ, Duman RS. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002; 22:3673–3682.

26. Nam SM, Kim JW, Yoo DY, Choi JH, Kim W, Jung HY, Won MH, Hwang IK, Seong JK, Yoon YS. Effects of treadmill exercise on neural stem cells, cell proliferation, and neuroblast differentiation in the subgranular zone of the dentate gyrus in cyclooxygenase-2 knockout mice. Neurochem Res. 2013; 38:2559–2569.

27. Paulson SK, Zhang JY, Breau AP, Hribar JD, Liu NWK, Jessen SM, Lawal YM, Cogburn JN, Gresk CJ, Markos CS, Maziasz TJ, Schoenhard GL, Burton EG. Pharmacokinetics, tissue distribution, metabolism, and excretion of celecoxib in rats. Drug Metab Dispos. 2000; 28:514–521.
28. Pinnock SB, Blake AM, Platt NJ, Herbert J. The roles of BDNF, pCREB and Wnt3a in the latent period preceding activation of progenitor cell mitosis in the adult dentate gyrus by fluoxetine. PLoS One. 2010; 5:e13652.

29. Rall JM, Mach SA, Dash PK. Intrahippocampal infusion of a cyclooxygenase-2 inhibitor attenuates memory acquisition in rats. Brain Res. 2003; 968:273–276.

30. Rettori V, Gimeno M, Lyson K, McCann SM. Nitric oxide mediates norepinephrine-induced prostaglandin E2 release from the hypothalamus. Proc Natl Acad Sci U S A. 1992; 89:11543–11546.

31. Roberson ED, English JD, Adams JP, Selcher JC, Kondratick C, Sweatt JD. The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J Neurosci. 1999; 19:4337–4348.

32. Rolando C, Taylor V. Neural stem cell of the hippocampus: development, physiology regulation, and dysfunction in disease. Curr Top Dev Biol. 2014; 107:183–206.
33. Russo I, Amornphimoltham P, Weigert R, Barlati S, Bosetti F. Cyclooxygenase-1 is involved in the inhibition of hippocampal neurogenesis after lipopolysaccharide-induced neuroinflammation. Cell Cycle. 2011; 10:2568–2573.

34. Sasaki T, Kitagawa K, Sugiura S, Omura-Matsuoka E, Tanaka S, Yagita Y, Okano H, Matsumoto M, Hori M. Implication of cyclooxygenase-2 on enhanced proliferation of neural progenitor cells in the adult mouse hippocampus after ischemia. J Neurosci Res. 2003; 72:461–471.

35. Serrano GE, Lelutiu N, Rojas A, Cochi S, Shaw R, Makinson CD, Wang D, FitzGerald GA, Dingledine R. Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus. J Neurosci. 2011; 31:14850–14860.

36. Sharifzadeh M, Tavasoli M, Soodi M, Mohammadi-Eraghi S, Ghahremani MH, Roghani A. A time course analysis of cyclooxygenase-2 suggests a role in spatial memory retrieval in rats. Neurosci Res. 2006; 54:171–179.

37. Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004; 56:387–437.

38. Teather LA, Packard MG, Bazan NG. Post-training cyclooxygenase-2 (COX-2) inhibition impairs memory consolidation. Learn Mem. 2002; 9:41–47.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download