Abstract
The objective of the present study was to investigate the effects of three different culture media on the development of canine somatic cell nuclear transfer (SCNT) embryos. Canine cloned embryos were cultured in modified synthetic oviductal fluid (mSOF), porcine zygote medium-3 (PZM-3), or G1/G2 sequential media. Our results showed that the G1/G2 media yielded significantly higher morula and blastocyst development in canine SCNT embryos (26.1% and 7.8%, respectively) compared to PZM-3 (8.5% and 0%) or mSOF (2.3% and 0%) media. In conclusion, this study suggests that blastocysts can be produced more efficiently using G1/G2 media to culture canine SCNT embryos.
In many mammalian species, somatic cell nuclear transfer (SCNT) embryos have been developed to blastocyst stage under optimized in vitro culture conditions. Several culture media, such as modified synthetic oviductal fluid (mSOF), have been used to culture bovine [5] and ovine [7] SCNT embryos. Porcine zygote medium-3 (PZM-3) has been used to culture porcine [1] SCNT embryos from the zygote to blastocyst stage. However, no reports on successful blastocyst development of canine SCNT embryos have been published. To date, only two investigations have been conducted regarding the in vitro development of canine SCNT embryos [613]. In these studies, canine SCNT embryos cultured in mSOF medium developed to the 6-cell and morula stages, indicating that the low development of canine SCNT embryos may be due to the use of sub-optimal culture media. Thus, the objective of the present study was to investigate the effects of three different culture media on the development of canine SCNT embryos.
This study was carried out in accordance with the Guidelines for the Care and Use of Laboratory Animals of the National Institute of Animal Science and was approved by the National Institute of Animal Science Institutional Animal Care and Use Committee (approval no. NIAS 2013-054). Adult fibroblasts were isolated from an ear skin biopsy of a Labrador retriever. Ear tissues were cultured in advanced Dulbecco's modified Eagle's medium (Gibco-BRL, USA) supplemented with 10% (v/v) fetal bovine serum (FBS) and an antibiotic-antimycotic mixture at 38.5℃ in 5% CO2 and 95% air. Fibroblast cells at passages from two to five were cultured to confluency for synchronization in the G0/G1 stage and used for SCNT. Recovery of oocytes matured in vivo and SCNT were performed as previously described [6]. In vivo matured canine oocytes were obtained by flushing the oviducts of mixed-breed female dogs, and cumulus cells of the recovered oocytes were removed by repeated pipetting in holding medium (HEPES-buffered tissue culture medium-199 supplemented with 10% FBS) containing 0.1% hyaluronidase. The matured oocytes were then stained with 5 µg/mL bisbenzimide (Hoechst 33342) for 5 min and enucleated with a micromanipulator in holding medium supplemented with 5 µg/mL cytochalasin B. A fibroblast cell with a smooth surface was injected into the perivitelline space of an enucleated oocyte. The couplets were placed in fusion medium (0.26 M mannitol, 0.1 mM MgSO4, 0.5 mM HEPES, and 0.05% [w/v] bovine serum albumin [BSA]) and fused by electrical stimulation (two DC pulses of 34 V for 15 µsec) delivered with electrical rods. After 30 min of electrical stimulation, fusion was confirmed by microscopic observation. Fused embryos were activated in mSOF medium containing 10 µM calcium ionophore for 4 min followed by culturing in mSOF medium supplemented with 1.9 mM 6-dimethylaminopurine for 4 h. The activated embryos were maintained in 20 µL microdrops of mSOF, PZM-3, or G1/G2 sequential media (Vitrolife, Sweden) covered with mineral oil at 38.5℃ in 5% O2, 5% CO2, and 90% N2 for 8 days. For culturing in G1/G2 sequential media, the embryos were first cultured in G1 medium for 3 days, and then further cultured in G2 medium for 5 days. All data was analyzed using a chi-square test. P values < 0.05 were considered significant.
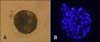
Our results showed that culturing canine SCNT embryos in G1/G2 media resulted in greater cleavage as well as morula and blastocyst development compared to PZM-3 or mSOF media (Table 1). Cleavage rates were higher (p < 0.05) for G1/G2 [n = 101/115 (87.8%)] and PZM-3 media [n = 40/47 (86.5%)] than mSOF media [n = 26/44 (59.1%)]. Development up to the morula or blastocyst stage was significantly higher (p < 0.05) for G1/G2 media [n = 30/115 (26.1%) and n = 9/115 (7.8%), respectively] compared to PZM-3 [n = 4/47 (8.5%) and n = 0/47 (0%), respectively] or mSOF media [n = 1/44 (2.3%) and n = 0/44 (0%), respectively]. The blastocysts cultured in G1/G2 media contained 59.0 ± 2.8 cells (range: 50~70, n = 8; Fig. 1).
Some media, such as TCM-199 [910] and mSOF [11], have been previously used for culturing canine embryos. However, these reagents are inadequate for culturing canine embryos in vitro due to very low developmental capacity of the resulting embryos into the blastocyst stage. A previous study demonstrated that one canine oocyte could develop to the blastocyst stage following in vitro fertilization (IVF) [10]. In the present investigation, three different media were used to determine the effect of the media on the development of canine SCNT embryos. mSOF medium has been used for culturing embryos for bovine IVF and SCNT embryos from the zygote to blastocyst stage [412]. PZM-3 medium has been widely utilized for culturing porcine embryos in most IVF and cloning experiments [1415]. G1/G2 sequential media are most widely used for human and mouse embryos [3] because these reagents accommodate the changing carbohydrate and amino acid requirements of the embryo and were specifically formulated to prevent intracellular stress in the embryo [812]. During the early cleavage stage, the embryo has a low metabolic activity with a limited ability to utilize glucose; it generates energy from the oxidation of pyruvate/lactate and non-essential amino acids at low levels [2]. Conversely, the post-compaction embryo has a high metabolic activity, uses glucose as the preferred nutrient, and requires nonessential along with essential amino acids for cell proliferation and differentiation as well as specific vitamins to maintain oxidation [2]. G1/G2 sequential media were formulated to mimic environmental changes experienced by the developing embryo in vivo [2].
In conclusion, results of the present study showed that G1/G2 sequential media is superior to PZM-3 and mSOF media for supporting the development of canine SCNT embryos in vitro. To the best of our knowledge, this study is the first to show that canine SCNT embryos can be developed to the blastocyst stage in vitro. Our data suggest that blastocyst embryos can be produced in vitro by culturing canine SCNT embryos in G1/G2 sequential media. However, further studies are needed to enhance development into blastocyst stage and improve the quality of SCNT-derived canine embryos.
Figures and Tables
Acknowledgments
This work was supported by the Cooperative Research Program for Agriculture Science and Technology Development (project no. PJ009333022014), Rural Development Administration, Korea.
References
1. Cao Z, Sui L, Li Y, Ji S, Zhang X, Zhang Y. Effects of chemically defined medium on early development of porcine embryos derived from parthenogenetic activation and cloning. Zygote. 2012; 20:229–236.

2. Garcia-Garcia RM, Ward F, Fair S, O'Meara CM, Wade M, Duffy P, Lonergan P. Development and quality of sheep embryos cultured in commercial G1.3/G2.3 sequential media. Anim Reprod Sci. 2007; 98:233–240.

3. Gardner DK, Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum Reprod Update. 1997; 3:367–382.

4. Holm P, Booth PJ, Schmidt MH, Greve T, Callesen H. High bovine blastocyst development in a static in vitro production system using SOFaa medium supplemented with sodium citrate and myo-inositol with or without serum-proteins. Theriogenology. 1999; 52:683–700.

5. Jang G, Hong SG, Lee BC. Cloned claves derived from somatic cell nuclear transfer embryos cultured in chemically defined medium or modified synthetic oviduct fluid. J Vet Sci. 2011; 12:83–89.

6. Jang G, Oh HJ, Kim MK, Fibrianto YH, Hossein MS, Kim HJ, Kim JJ, Hong SG, Park JE, Kang SK, Lee BC. Improvement of canine somatic cell nuclear transfer procedure. Theriogenology. 2008; 69:146–154.

7. Khan FA, Bhat MH, Yaqoob SH, Waheed SM, Naykoo NA, Athar H, Khan HM, Fazili MR, Ganai NA, Singla SK, Shah RA. In vitro development of goat-sheep and goat-goat zona-free cloned embryos in different culture media. Theriogenology. 2014; 81:419–423.

8. Lane M, Gardner DK, Hasler MJ, Hasler JF. Use of G1.2/G2.2 media for commercial bovine embryo culture: equivalent development and pregnancy rates compared to co-culture. Theriogenology. 2003; 60:407–419.

9. Lee SR, Kim BS, Kim JW, Kim MO, Kim SH, Yoo DH, Shin MJ, Park YS, Lee S, Park YB, Ha JH, Ryoo ZY. In vitro maturation, in vitro fertilization and embryonic development of canine oocytes. Zygote. 2007; 15:347–353.

10. Otoi T, Murakami M, Fujii M, Tanaka M, Ooka A, Une S, Suzuki T. Development of canine oocytes matured and fertilised in vitro. Vet Rec. 2000; 146:52–53.
11. Rodrigues Bde A, dos Santos LC, Rodrigues JL. Embryonic development of in vitro matured and in vitro fertilized dog oocytes. Mol Reprod Dev. 2004; 67:215–223.
12. Wang YS, Tang S, An ZX, Li WZ, Liu J, Quan FS, Hua S, Zhang Y. Effect of mSOF and G1.1/G2.2 media on the developmental competence of SCNT-derived bovine embryos. Reprod Domest Anim. 2011; 46:404–409.

13. Westhusin ME, Burghardt RC, Ruglia JN, Willingham LA, Liu L, Shin T, Howe LM, Kraemer DC. Potential for cloning dogs. J Reprod Fertil Suppl. 2001; 57:287–293.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download