Abstract
In the present study, 13 clinical cases of canine mammary adenocarcinoma were evaluated in order to understand the effect of Tarantula cubensis extract (TCE) on tumor tissue. Punch biopsies were taken from the tumors before treatment with TCE. Subcutaneous injections of TCE were administered three times at weekly intervals (3 mL per dog). Between days 7 and 10 after the third injection, the tumor masses were extirpated by complete unilateral mastectomy. Pre- and post-treatment tumor tissues were immunohistochemically assessed. The expression of B-cell lymphoma 2 (Bcl-2) was found to be higher in pre-treatment compared to post-treatment tissues (p < 0.01) whereas Ki-67 expression was lower in post-treatment tissues (p < 0.01). No significant differences in fibroblast growth factor or vascular endothelial growth factor expression were observed between pre- and post-treatment tissues (p > 0.05). The apoptotic index was determined to be low before treatment and increased during treatment. These results suggest that TCE may be effective for controlling the local growth of canine mammary adenocarcinoma by regulating apoptosis.
Mammary gland tumors are the second most common tumor type in female dogs next to skin tumors [17]. Nearly half of canine mammary tumors (CMTs) are diagnosed as malignant [24]. Although first-line treatment of tumors is primarily surgical excision [23], combined approaches including chemical and/or radiation therapy are often required since malignant tumors are able to infiltrate surrounding tissues and metastasize [8].
Recently, studies of growth factors and enzymes gave more insight into the molecular characteristics of different cancer types. [26] Tumor markers can be used to detect tumor activity, tumor cell type, response to therapy, or recurrence. Some markers, such as anti-apoptosis factors, are used to determine if a tumor is susceptible to specific therapies or to follow-up the proliferative stage of a tumor such as with Ki-67 [26].
Ki-67 antigen is a highly protease-sensitive nuclear protein that is expressed during all phases of cycling cells except for G0 [12]. This factor is considered a good marker for cell proliferation [5]. Therefore, Ki-67-specific immunostaining is an alternative method for assessing proliferative activity in mammary gland tumors, and produces a reaction with a nuclear antigen found during several cell cycle phases (G1, S, and G2) as well as mitosis [12]. Ki-67 expression was found to be higher in malignant tumors than in nonmalignant tumors [38].
B-cell lymphoma 2 (Bcl-2) is an oncoprotein that plays a role in apoptosis by blocking programmed cell death [116]. Its expression is related to the expression of steroid hormone receptors and low tumor proliferative activity in human breast cancer [37]. Additionally, levels of this protein were found to be higher in benign than malignant tumors [37].
The formation of vascular stroma influences the growth since a tumor will not be able to expand beyond 1~2 mm without angiogenesis. Vascular endothelial growth factor (VEGF), an endothelial mitogen, was initially recognized for its ability to increase microvascular permeability. This protein is a multifunctional cytokine that exerts effects on vascular endothelium features such as endothelial mitogenesis and permeability [4]. It was shown that VEGF is overexpressed in mammary gland tumors [4] and may contribute to tumor growth, invasion, and metastasis into surrounding microvessels via autocrine pathways [34]. It was also determined that VEGF expression in mammary gland tumors is higher than that in healthy canine mammary glands [29].
Fibroblast growth factor (FGF) is a mitogen for fibroblasts and epithelial cells. This factor is also a strong promoter of angiogenesis. FGF receptors were shown to be overexpressed both in human and animal mammary tumors [14].
Tarantula cubensis extract (TCE) has been found to stop the growth of CMTs by promoting demarcation from surrounding tissues [19]. TCE is used as a homeopathic remedy for treating inflammation in the skin, connective tissue, and nervous system. It is very effective for ameliorating painful abscesses, inflammatory ulcers, and severe dermatitis [15]. TCE also resulted in regression of benign mammary tumors and hardness in malignant mammary tumors. [13].
The purpose of the current study was to evaluate the effects of TCE on canine mammary adenocarcinomas. For this, the expression of Bcl-2, Ki-67, VEGF, and FGF was evaluated with immunohistochemical staining and a terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling (TUNEL) assay. To the best of our knowledge, this study is the first to examine the effects of TCE on canine mammary tumors.
Spontaneous CMTs brought to the clinic were used for this study. To obtain a preferably homogenous treatment group, only animals with a comparable case history and clinical as well as histological findings were included in the investigation. Routine diagnosis and treatment of mammary tumors including taking biopsies, homeopathic regulation treatment with TCE, and mastectomy were performed for all canines. The dogs were privately owned and lived in households. In addition, the owners were informed that the TCE used in the study is known to inhibit the growth of CMTs [19]. In cases of multiple mammary tumors, only the largest was able to be biopsied; therefore, the behavior of each tumor before and after TCE treatment could not have been assessed since mammary tumors are frequently multicentric and pathologically separate entities. In addition, a control group was not established since performing a mastectomy on healthy dogs is not ethical according to the law in Turkey. The research was conducted in accordance with the principles outlined in decision no. 2009-12 of the Ethical Committee of Animal Research of Turkey.
Thirteen intact female dogs brought to the hospital with a history of mammary gland masses were included in the investigation. The breeds of the dogs were three Norfolk terriers, three bichon Havanese, five mongrels, one cocker spaniel, and one Doberman pinscher (Table 1). The animal ages ranged from 7 to 14 years, and none of the dogs had a history of any treatment for mammary tumors. Seven dogs had multiple tumors on the same mammary gland chain. Staging of the mammary tumors was performed according to the World Health Organization (WHO) Clinical Staging System TNM [27]. In cases of multiple tumors, the largest one was used for classification. Tumor size was measured, the consistency and degree of demarcation palpated, and the results were compared to the findings observed after treatment (+ to +++). Metastasis screening was done using thoracic radiographs and ultrasonographic examination of the abdominal organs.
After tumor staging, punch biopsies were taken using a single-use biopsy punch (6 mm; Kai Medical, Japan). In cases of multiple mammary tumors, only the largest one was biopsied. The hardest part of the tumors was chosen to ensure that the lesion was evaluated. Before sampling, the hair was thoroughly clipped and the skin was sterilized with alcohol. Sedation or local anesthesia was not induced. After excision of samples with a minimum diameter of 5 mm, suturing was not required and low-grade hemorrhaging was stopped by pressing a swab to the wound. In all patients, none of the small wounds showed complications during healing within the following week.
TCE was injected between the shoulder blades (Theranekron : D2 = 1 : 100; Richter Pharma, Austria) three times at weekly intervals. The dosage was 3 mL per dog as indicated in the prospectus. Between days 7 and 10 after the third injection, the patients underwent complete unilateral mastectomy. All dogs in the study received a follow-up examination during the 18 months after mastectomy.
Both the tumors and biopsies were immediately fixed in 10% neutral buffered formalin and embedded in paraffin. From all tissue samples, 5-µm sections were cut and stained with hematoxylin and eosin. The biopsies and tumors were evaluated by the same pathologist and classified histologically according to the WHO International Classification system [25].
Additional sections placed on 3-aminopropyltriethoxysilane (Sigma, USA)-coated slides were stained using the streptavidin-biotin-peroxidase complex technique (Histostain Plus Kit; ZYMED, USA) with monoclonal anti-VEGF antibody (SC-7269; Santa Cruz Biotechnology, USA), anti-Bcl-2 antibody (SC-7382; Santa Cruz Biotechnology), anti-Ki-67 antibody (MIB-1; Dako, Denmark), and polyclonal anti-FGF antibody (SC-79; Santa Cruz Biotechnology). Antigen retrieval was performed by heating the sections in citrate buffer (pH 6.0) for 20 min in a microwave oven at 600 W. Aminoethylcarbazole or 3,3-diaminobenzidine in H2O2 were used as chromogens with incubation for 10 min (controlled by visual observation under a microscope). The sections were counterstained with Mayer's hematoxylin for 1 min, rinsed with tap water, and mounted with an aqueous mounting medium. The percentages of the total area of the positive cells were determined using a microscopy image analysis system (Bs200P Image Analysis System; BAB Software, Turkey). A total of 10 high-power fields were randomly chosen and analyzed at 400× magnification.
To assess the degree of DNA fragmentation, the sections were stained using the TUNEL method (in situ cell death detection kit; Roche Diagnostics, Germany) according to the manufacturer's instructions. The sections were de-paraffinized and rehydrated. Next, the sections were irradiated at 350 W in 0.1 µm citrate buffer (pH 6.0) in a microwave oven for 5 min. After washing twice in PBS (pH 7.4), the sections were covered with 50 µL of the TUNEL reaction mixture containing terminal deoxynucleotidyl transferase and fluorescein-dUTP (2'-deoxyuridine 5'-triphosphate), and incubated under a coverslip in a humidified chamber for 1 h at 37℃. The slides were next incubated with horseradish peroxidase-conjugated anti-fluorescein antibody for 40 min at room temperature. After washing three times for 15 min each in PBS, the sections were finally incubated with the chromogenic substrate 3,3-diaminobenzidine for 5 to 15 min at room temperature.
Initial physical examination results indicated that all dogs were in a good state of health. Average tumor size was 43.93 ± 26.53 mm. All tumors were smooth with good demarcation (++). Regional lymph nodes were not enlarged. Metastases were not detected on thoracic radiographs or during abdominal ultrasonographic examinations. Based on these findings, all tumors were comparable and classified as T2N0M0 (Stage II). This staging was confirmed by histopathologic examination after complete unilateral mastectomy including the axillar and inguinal lymph nodes.
Seven days after the third TCE injection, average tumor size was 33.14 ± 23.42 mm. No significant difference between the pre- and post-treatment period was observed (p > 0.05). During initial examination, the primary tumors were slightly soft. The first and second injections of TCE did not exert any visible or measurable effect on the tumors. However, the examination performed on day 7 following the third injection revealed that demarcation of the tumor was increased and the skin covering the tumor was difficult to lift. A capsule-like structure was palpated around the tumor and the consistency of the tumor was harder than that observed before the injection. The dogs underwent complete unilateral mastectomy 7~10 days after the third TCE injection. No complications related to surgery were observed, and wound healing was per primam intentionem within the following 7~10 days. During the post-surgical follow-up period of 18 months, new tumor formation on the incision line or on the other mammary glands was not detected. The general condition of the dogs was good based on the clinical examinations performed monthly.
According to evaluation of the biopsies and tumors, the dogs were diagnosed with adenocarcinomas. Histopathological evaluation revealed that the tumors were completely removed in all cases. Subclassification was not performed since a limited number of animals was included in the study.
The mean percentages of areas positive for Ki-67, Bcl-2, VEGF, FGF, and apoptosis are shown in Fig. 1. Expression of Ki-67 (representing mitotic activity) and Bcl-2 (an anti-apoptotic protein) was found to be significantly decreased in the post-treatment tissues compared to the pre-treatment tissues (p < 0.01; Fig. 2). Furthermore, the TUNEL assay data revealed that significantly more apoptotic cells were present in the post-treatment tissues than in the pre-treatment tissues (p < 0.01; Fig. 3). However, there was no significant difference in VEGF or FGF expression between the pre- and post-treatment tissues (p > 0.05; Fig. 4).
Our study revealed that treatment with TCE led to a significant decrease in the expression of Ki-67 and Bcl-2 (p < 0.01; panels A-D in Fig. 2). Ki-67, one of the proliferation-associated nuclear antigens, is used to measure growth in CMTs [22]. It was previously observed that benign tumors have lower numbers of Ki-67-positive cells compared to malignant tumors even though the values did not differ between simple and complex carcinomas [35]. In another study, strong Ki-67 expression was detected in a mammary adenocarcinoma in a female dog [28]. Other studies showed that Ki-67 proliferation indices were higher for malignant than benign mammary tumors in female canines [3811], and the Ki-67 index was found to correlate with CMT prognosis. Ki-67 (MIB-1) reacts with a nuclear antigen present during the G1, S, and G2 phases of the cell cycle but is absent during G0 [11]. Furthermore, the MIB-1 index is an important prognostic factor as it can be used to determine the number of cycling cells [33]. In the present study, decreased Ki-67 expression observed after treatment of CMTs with TCE for 3 weeks revealed that the number of cycling cells was reduced or the proliferation rate decreased. Measurement of Ki-67 antigen expression thus helped to determine the response of tumor tissues to the TCE treatment.
As an important regulator of apoptosis, Bcl-2 is a frequently studied gene in cases of human breast cancer [20]. It was found that Bcl-2 expression in human breast adenocarcinomas is strongly related to hormone receptor positivity and low histologic grade [3]. Bcl-2 expression is also significantly increased in both human and canine mammary tumors [21]. In one previous study, the amount of Bcl-2 expression was determined to be 67% in benign and 58% in malignant CMTs [37]. In our study, Bcl-2 expression was significantly lower in post-treatment tissues compared to pre-treatment tissues (p < 0.01).
In humans, Bcl-2 expression was analyzed to characterize its relationship with patient outcomes in cases of breast cancer. It was discovered that Bcl-2 overexpression is related to a favorable prognosis in patients receiving hormonal or cytotoxic therapies [7] and is therefore believed to be a predictor of treatment response [1018]. Furthermore, patients with Bcl-2-positive tumors were found to have relapse-free survival rate three times lower than that of individuals with tumors lacking Bcl-2 expression [7].
In another study conducted to identify predictive markers related to chemotherapy sensitivity, Bax-positive and Bcl-2-negative breast cancer was found to have a significantly higher reduction rate per chemotherapy cycle [34]. In addition, antitumor effects of Curcuma phaeocaulis Valeton (CpV) extract were evaluated using MCF-7 and MDA-MB-231 breast cancer cells. It was discovered that CpV, a Chinese medical herb, significantly inhibits cell proliferation, induces apoptosis, and decreases the expression of Bcl-2 [6]. Similarly, we showed that the expression of Ki-67 and Bcl-2 was lower following treatment with TCE. We also determined that the administration of TCE induced apoptosis. Conditions that cause up-regulation of cell proliferation or suppression of apoptosis are known to result in mutations and breast cancer development [20]. Overexpression of Bcl-2 was found to suppress apoptosis [9]. We therefore hypothesize that TCE induced apoptosis by decreasing Bcl-2 expression in our investigation.
In the present study, the expressions of VEGF and FGF were measured since these factors are markers of angiogenesis [1431]. However, no significant differences were detected between pre- and post-treatment tumor tissues (p > 0.05). Involvement of FGF receptors has been identified both in human carcinogenesis and animal mammary tumors [14]. Many studies previously demonstrated VEGF overexpression in high-grade canine malignant tumors [23031] and its correlation with metastasis [3236]. On the other hand, a recent investigation showed that VEGF expression does not differ significantly between benign and malignant CMTs [32]. According to this study, VEGF expression is independent of histological type, cell proliferation, and metastatic capacity. It was also determined that normal mammary epithelial cells express VEGF, suggesting that stages of the oestrus cycle may promote cellular proliferation and increase the vascular network [32].
Down-regulation of VEGF expression as a way to decrease angiogenesis is a goal for cancer treatment. However, TCE did not affect VEGF expression in canine mammary adenocarcinomas in the present investigation. Further studies are therefore required to better understand the regulation of VEGF expression and evaluate the effects of anti-angiogenic drugs such as anti-VEGF antibodies, endostatin, and angiostatin together with TCE treatment. We found that TCE treatment for 3 weeks increased the consistency of the primary tumor. Similary, three injections of 3~6 mL TCE given 7 days apart has been reported to stop visible growth of mammary tumors in female dogs within 2~4 weeks [19]. The tumors became harder and demarcation from the surrounding tissue increased, thereby facilitating surgical intervention [19]. In our study, we tried to correlate this observation with the expression of FGF but no relationship could be detected. A primary objective of cancer treatment is to enhance apoptosis and reduce cell proliferation. The high levels of Bcl-2 and Ki-67 expression observed in the pre-treatment tissues and reduced levels in the post-treatment tissues indicated that the administration of TCE may help control tumor growth in canine mammary adenocarcinomas classified as T2N0M0.
Figures and Tables
Fig. 1
Mean percentage of areas positive for Bcl-2 (p < 0.01), Ki-67 (p < 0.01), apoptosis (p < 0.01), vascular endothelial growth factor (VEGF; p > 0.05), and fibroblast growth factor (FGF; p > 0.05).

Fig. 2
Immunohistochemical staining for Ki-67 and B-cell lymphoma 2 (Bcl-2). (A) Pre-treatment Ki-67-positive cells (arrow). (B) Post-treatment Ki-67-positive cells (arrow). (C) Pre-treatment Bcl-2-positive cells (arrow). (D) Post-treatment Bcl-2-positive cells. Immunoperoxidase technique with Mayer's haematoxylin counterstaining. Magnification: 240× (A), 180× (B and C), 140× (D).
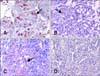
Fig. 3
Pre- and post-treatment terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling (TUNEL) results. (A) Post-treatment TUNEL-positive apoptotic cells (arrow). (B) Pre-treatment TUNEL-positive apoptotic cells (arrow). TUNEL assay with Mayer's haematoxylin counterstaining, Magnification: 240× (A), 140× (B).

Fig. 4
Immunohistochemical staining for VEGF and FGF. (A) Pre-treatment VEGF-positive cells (arrow). (B) Post-treatment VEGF-positive cells (arrow) (C) Pre-treatment FGF-positive cells (arrow). (D) Post-treatment FGF-positive cells (arrow). Immunoperoxidase technique with Mayer's haematoxylin counterstaining, Magnification: 180× (A and B), 140× (C and D).
Acknowledgments
The authors would like to thank Richter Pharma (Austria) and Interhas A.S. (Turkey) for providing the antibodies and TCE.
References
1. Abbas AK, Lichtman AH. Cellular and Molecular Immunology. 5th ed. Philadelphia: Saunders;2003. p. 216–240.
2. Al-Dissi AN, Haines DM, Singh B, Kidney BA. Immunohistochemical expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 in canine simple mammary gland adenocarcinomas. Can Vet J. 2010; 51:1109–1114.
3. Alsabeh R, Wilson CS, Ahn CW, Vasef MA, Battifora H. Expression of bcl-2 by breast cancer: a possible diagnostic application. Mod Pathol. 1996; 9:439–444.
4. Bamberger ES, Perrett CW. Angiogenesis in epithelian ovarian cancer. Mol Pathol. 2002; 55:348–359.

5. Castagnaro M, De Maria R, Bozzetta E, Ru G, Casalone C, Biolatti B, Caramelli M. Ki-67 index as indicator of the post-surgical prognosis in feline mammary carcinomas. Res Vet Sci. 1998; 65:223–226.

6. Chen X, Pei L, Zhong Z, Guo J, Zhang Q, Wang Y. Anti-tumor potential of ethanol extract of Curcuma phaeocaulis Valeton against breast cancer cells. Phytomedicine. 2011; 18:1238–1243.

7. Daidone MG, Luisi A, Veneroni S, Benini E, Silvestrini R. Clinical studies of Bcl-2 and treatment benefit in breast cancer patients. Endocr Relat Cancer. 1999; 6:61–68.

8. Dobson JM, Gorman NT. Cancer Chemotherapy in Small Animal Practice. Oxford: Blackwell Scientific;1993.
9. Dole M, Nuñez G, Merchant AK, Maybaum J, Rode CK, Bloch CA, Castle VP. Bcl-2 inhibits chemotherapy-induced apoptosis in neuroblastoma. Cancer Res. 1994; 54:3253–3259.
10. Gee JM, Robertson JF, Ellis IO, Willsher P, McClelland RA, Hoyle HB, Kyme SR, Finlay P, Blamey RW, Nicholson RI. Immunocytochemical localization of bcl-2 protein in human breast cancers and its relationship to a series of prognostic markers and response to endocrine therapy. Int J Cancer. 1994; 59:619–628.

11. Geraldes M, Gärtner F, Schmitt F. Immunohistochemical study of hormonal receptors and cell proliferation in normal canine mammary glands and spontaneous mammary tumours. Vet Rec. 2000; 146:403–406.

12. Gerdes J, Li L, Schlueter C, Duchrow M, Wohlenberg C, Gerlach C, Stahmer I, Kloth S, Brandt E, Flad HD. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol. 1991; 138:867–873.
13. Gültiken N, Vural MR. The effect of Tarantula cubensis extract applied in pre and postoperative period of canine mammary tumours. J Istanb Vet Sci. 2007; 2:13–23.
14. Halper J. Growth factors as active participants in carcinogenesis: a perspective. Vet Pathol. 2010; 47:77–97.

15. Hamilton D. Homeopathic Care for Cats and Dogs: Small Doses for Small Animals. Berkley: North Atlantic Books;1999. p. 237–272.
16. Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990; 348:334–336.

17. Johnston SD, Kustritz MVR, Olson PNS. Canine and Feline Theriogenology. 1st ed. Philadelphia: Saunders;2001. p. 243–256.
18. Keen JC, Dixon JM, Miller EP, Cameron DA, Chetty U, Hanby A, Bellamy C, Miller WR. The expression of Ki-S1 and BCL-2 and response to primary tamoxifen therapy in elderly patients with breast cancer. Breast Cancer Res Treat. 1997; 44:123–133.

19. Koch H, Stein M. Conservative treatment of neoplasms of the mammary gland in bitches, with the use of Theranekron. Prakt Tierarzt. 1980; 61:429–430.
20. Kumar R, Vadlamudi RK, Adam L. Apoptosis in mammary gland and cancer. Endocr Relat Cancer. 2000; 7:257–269.

21. Kumaraguruparan R, Prathiba D, Nagini S. Of humans and canines: immunohystochemical analysis of PCNA, Bcl-2, p53, cytokeratin and ER in mammary tumours. Res Vet Sci. 2006; 81:218–224.

22. Löhr CV, Teifke JP, Failing K, Weiss E. Characterization of the proliferation state in canine mammary tumors by the standardized AgNOR method with postfixation and immunohistologic detection of Ki-67 and PCNA. Vet Pathol. 1997; 34:212–221.

23. MacEwen EG, Withrow SJ. Tumors of the mammary gland. In : Withrow SJ, MacEwen EG, editors. Clinical Veterinary Oncology. Philadelphia: Lippincott;1989. p. 292–304.
24. Misdorp W. Tumors of the mammary gland. In : Meuten DJ, editor. Tumors in Domestic Animals. 4th ed. Ames: Iowa State Press;2002. p. 575–606.
25. Misdorp W, Else RW, Hellmén E, Lipscomb TP. Histological Classification of Mammary Tumors of the Dog and Cat. 2nd ed. Washigton D.C.: Armed Forces Institute of Pathology;1999. p. 1–59.
26. Osborne CA. Generating the diagnosis and prognosis of cancer in geriatric pets. In : Villalobos A, Kaplan L, editors. Canine and Feline Geriatric Oncology: Honoring the Human-Animal Bond. 1st ed. Ames: Blackwell;2007. p. 103–134.
27. Owen LN. TNM Classification of Tumours in Domestic Animals. 1st ed. Geneva: World Health Organization;1980.
28. Ozmen O, Haligur M, Kocamuftuoglu M. Clinocopathologic and immunohistochemical findings of multiple genital leiomyomas and mammary adenocarcinomas in a bitch. Reprod Domest Anim. 2008; 43:377–381.

29. Qiu C, Lin DD, Wang HH, Qiao CH, Wang J, Zhang T. Quantification of VEGF-C expression in canine mammary tumours. Aust Vet J. 2008; 86:279–282.

30. Qiu CW, Lin DG, Wang JQ, Li CY, Deng GZ. Expression and significance of PTEN and VEGF in canine mammary gland tumours. Vet Res Commun. 2008; 32:463–472.

31. Restucci B, Papparella S, Maiolino P, De Vico G. Expression of vascular endothelial growth factor in canine mammary tumours. Vet Pathol. 2002; 39:488–493.

32. Santos AA, Oliveira JT, Lopes CCC, Amorim IF, Vicente CMFB, Gärtner FRM, Matos AJF. Immunohistochemical expression of vascular endothelial growth factor in canine mammary tumours. J Comp Pathol. 2010; 143:268–275.

33. Sarli G, Preziosi R, Benazzi C, Castellani G, Marcato PS. Prognostic value of histologic stage and proliferative activity in canine malignant mammary tumors. J Vet Diagn Invest. 2002; 14:25–34.

34. Tewari M, Pradhan S, Singh U, Singh TB, Shukla HS. Assesment of predictive markers of response to neoadjuvant chemotherapy in breast cancer. Asian J Surg. 2010; 33:157–167.

35. Thuróczy J, Reisvaag GJ, Perge E, Tibold A, Szilágyi J, Balogh L. Immunohistochemical detection of progesteron and cellular proliferation in canine mammary tumours. J Comp Pathol. 2007; 137:122–129.

36. Weidner N. Tumoural vascularity as a prognostic factor in cancer patients: the evidence continues to grow. J Pathol. 1998; 184:119–122.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download