Introduction
Animal models of arthritis are used to study pathogenesis of the disease and promote the development of therapeutics [2]. Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by persistent synovitis, systemic inflammation, and autoantibody production [19]. RA therapeutics reduce joint inflammation, limit erosive damage, and improve the quality of life [18].
The collagen-induced arthritis (CIA) model is the most commonly studied autoimmune model of RA. This type of autoimmune arthritis is induced by immunization with an emulsion of complete Freund's adjuvant and type II collagen [4]. CIA-induced lesions in rats represents gradual and progressive degeneration of articular cartilage, subchondral bone, and synovium as well as severe infiltration by inflammatory cells such as neutrophils, T lymphocytes [35], and macrophages [13].
Although pathologic evaluation is advantageous, this technique provides qualitative or semi-quantitative information but has a limited ability for the quantification of lesions. In addition, pathological evaluation of RA progression can only be performed after the animal has been sacrificed. Furthermore, this method may not be suitable for carrying out longitudinal or serial examinations.
Imaging techniques can be used to assess the efficacy and toxicity of developing pharmaceuticals, and help monitor the response of joint lesions. It was also reported that these techniques help reduce the number of animals required per experiment and provide increased statistical power; since each animal can function as its own control over time [6]. By facilitating longitudinal studies, imaging of animals has allowed continuous, dynamic, and sometimes nearly instantaneous identification and/or quantification of disease progression [12]. Thus, it is highly feasible to perform non-invasive imaging techniques to study animal disease models. Additionally, these methods are required for longitudinal studies to evaluate disease progression and response to therapy in live animals. One of these imaging modalities, micro-computed tomography (micro-CT) produces detailed anatomical images of animals [17].
Until now, combinational analyses involving micro-CT and conventional pathological methods for monitoring arthritis in rats have not been assessed. Therefore, the purpose of the present study was to perform a comprehensive characterization of rat CIA-induced arthritis using micro-CT. The results were to compared to data from a conventional pathological examination.
Materials and Methods
CIA model
Twenty-four female Wistar rats (8 weeks old) were obtained from Samtaco Bio (Korea) and acclimated for 7 days before the study was initiated. The animals were kept in a temperature-controlled environment (22 ± 3℃), with 55 ± 5% relative humidity and a 12-h light/dark cycle. The rats were fed a gamma ray-irradiated rodent diet (Cargill Agri Purina, Korea) and filtered water ad libitum.
The rats were randomly divided into four equal groups based on time points: week 0, 2, 3, and 4. Each rat was injected with an emulsion to induce arthritis except for the week 0 group. CIA was induced as follows. Bovine type II collagen (Chondrex, USA) was dissolved overnight in 0.01 M acetic acid at 4℃. This was emulsified in an equal volume of incomplete Freund's adjuvant (Chondrex). The rats were immunized intradermally at the base of the tail with 0.1 mL of emulsion containing 100 µg of type II collagen. On day 7 after primary immunization, the rats were boosted using the same technique that was used for the first round of injections. The animals were sacrificed at week 0 as well as week 2, 3, and 4 after the initial collagen immunization. All hind knee joints were fixed in 10% neutral phosphate-buffered formalin. This study was approved by the animal ethics committee of Namseoul University (Cheonan) according to the Animal Protection Act.
Micro-CT analysis
Quantitative analysis of the hind knee joints was performed using a micro-CT system (SKYSCAN 1172; Bruker, Belgium). Specimens were scanned with an X-ray source of 40 kV/250 µV, pixel size of 23 µm, and a 0.5 mm aluminum filter. After scanning, cross-sectional slices were generated and each scan result was reconstructed using 0~0.14 threshold values to distinguish bone from air.
Three-dimensional analysis was performed using CTAn software (Bruker). The fraction of bone volume (BV), percent bone volume (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), bone surface/bone volume (BS/BV), and trabecular separation (Tb.Sp) were evaluated at weeks 0, 2, 3, and 4 using the built-in software. In addition, osteophytes within each contiguous coronal image section were manually outlined and the volume was calculated using CTvol software (Bruker).
Pathological assessment
The hind knee joints were fixed in 10% phosphate-buffered formalin for 24 h and decalcified in 14% EDTA-glycerol for 14 days at room temperature. The specimens were routinely processed, embedded in paraffin, and cut into 4-µm sections that were stained with hematoxylin and eosin (H&E). To examine the cartilage, safranin O-fast green staining of the hind knee joints was performed.
Pathological analyses of the joints were carried out by monitoring infiltration of inflammatory cells, cartilage degradation, synovial proliferation, and chondrocyte hyperplasia. Lesion of severity was was classified according to four grades: 0, no detectable change; 1, slight; 2, moderate; 3, severe.
Results
CIA model
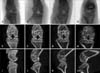
X-ray, coronal, and sagittal micro-CT images showed that the joints had a normal appearance at week 0. Joint destruction was observed in the collagen-treated rats at week 2, 3, and 4. Additionally, destruction of bony surfaces was increased at weeks 2, 3, and 4 in a time-dependent manner (Fig. 1).
Several bone parameters were altered during the development of arthritis according to the micro-CT findings (Fig. 2). BV was significantly decreased in the tibia of the rats at weeks 3 and 4 compared to week 0 (p < 0.05 and p < 0.01, respectively). BV/TV was significantly reduced in the tibia at week 4 compared to week 0 (p < 0.05). In contrast, BS/BV and Tb.Sp were significantly increased in the tibia at week 4 compared to week 0 (p < 0.05). Tb.N and Tb.Th, both tended to decrease in a time-dependent manner.
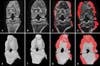
Using CTvol software, osteophytes in the hind knee joint of collagen-treated rats were visualized. Osteophyte development appeared as red coloring surrounding the irregular bone surface at weeks 3 and 4 (Fig. 3). Quantitative assessment of osteophytes over the knee was performed and calculated osteophyte volumes in the knee joints were 0 at week 0, 0 at week 2, 2.2 ± 3.8 at week 3, and 3.4 ± 5.8 at week 4.
Pathological observation
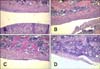
Time-dependent pathological alterations were observed in the rat knee joints. No inflammation or tissue destruction was found at week 0. However, knee joints of the collagen-treated rats showed severe joint destruction with inflammatory cell infiltration. This was accompanied by erosion of cartilage and bone along with the appearance of enlarged cavities filled with synovial fibroblasts and inflammatory cells in a time-dependent manner (Fig. 4).
Safranin O-fast green staining demonstrated that the joints of the rats had normal cartilage structures (indicated by red pigmentation) at week 0. However, significant cartilage destruction appeared in time-dependent manner in the joints of rats treated with collagen (Fig. 5).
Scoring of the pathological findings, revealed that infiltration of inflammatory cells, cartilage degradation, synovial proliferation, and hyperplasia of chondrocytes, increased in a time-dependent manner (Fig. 6).
Discussion
The current study showed that micro-CT analysis could be performed to evaluate several quantitative parameters of CIA, and these data generally correlated to microscopic finding.
Several bone parameters were altered during arthritis development according to micro-CT analyses that showed progressive bony structure changes at weeks 2, 3, and 4. BV was significantly decreased at weeks 3 and 4 while BV/TV was significantly reduced at week 4 compared to week 0. Both Tb.N and Tb.Th tended to decrease in a time-dependent manner. These data indicated that bone destruction was evident in rats treated with collagen at weeks 3 and 4. As BS/BV and Tb.Sp were significantly increased at week 4 compared to week 0, there could be the deposition of osteophytes around the surface and the evidence of bone loss.
Micro-CT can measure osteophytes formation as well as trabecular bone structures [12]. Therefore, we quantified osteophytes volumes in the knee joints (0, 0, 2.2 ± 3.8, and 3.4 ± 5.8 at weeks 0, 2, 3, and 4, respectively). Even though osteophytes volumes increased, this characteristic varied widely among the animals.
In the present study, cartilage destruction was observed. Safranin O-fast green staining showed that the joints of the rats represented normal cartilage structures at week 0. Significant cartilage destruction appeared in the joints of the collagen-treated animals in a time-dependent manner. Since the ability of micro-CT analysis to detect cartilage is limited, other imaging techniques should be performed. High-resolution magnetic resonance imaging (MRI) has been found to be sensitive to changes in cartilage integrity in rats [9]. It was also reported that MRI assessment of cartilage degeneration correlated to macroscopic grading [1]. Additionally, imaging devices such as positron emission tomography and single photon emission computed tomography can be applied to assess arthritis [612]. Furthermore, it was reported that intravital microscopy is a powerful tool for measuring the functionality and dynamics of individual regulators of cell migration [7]. Since collagenases, matrix metalloproteinase, or gelatinases promote joint destruction in cases of arthritis [10], quantitative analysis of the breakdown products of matrix components in joints is important for evaluating the effects of inflammatory joint disease [15]. Using a matrix metalloproteinase 3-specific polymeric probe, an early diagnosis of arthritis and visualization of arthritis progression and this approach could be used for early diagnosis as well as monitoring the effects of drug and surgical therapies [16]. Further studies are warranted to monitor animals treated with pharmaceuticals using in situ imaging modalities such as MRI and intravital microscopy as well as micro-CT.
In this investigation, HE staining showed that inflammatory cells invaded the synovium, sometimes forming a fibrovascular pannus eroding articular cartilage and subchondral bone in collagen-treated animals, in a time-dependent manner. Hypertrophy of the synovial lining is the first histological manifestation of early lesions associated with arthritis and mild inflammatory infiltrate into the synovium rapidly forms a fibrovascular pannus [11]. When monitoring cartilage degradation, highly varied pathological scores were obtained even at week 4. Immunization with type II collagen leads to the development of severe polyarticular arthritis in rodents that is mediated by an autoimmune response [14]. In cases of RA, macrophages are responsible for inducing inflammation, matrix destruction, and angiogenesis [13]. Further studies are needed to analyze the types of immune cells involved in the process of arthritis development in rats.
For semi-quantitative analysis of the pathological findings, infiltration of inflammatory cells, cartilage degradation, synovial proliferation, and hyperplasia/hypertrophy were scored. Even though infiltration of inflammatory cells, cartilage degradation, synovial proliferation, and hyperplasia tended to increase over time, none of the changes observed at weeks 2, 3, and 4 were significant compared to week 0. This is probably due to animal variation. Even though the scoring paradigm is sufficiently sensitive to evaluate cases of arthritis [8], it seems to be difficult to show statistic difference of these lesions in this study.
Based on our findings, we concluded that micro-CT is capable of characterizing degenerative changes, associated with arthritis that were detected by pathological examination. Development of an image-based model of RA using rats with CIA will be of great use to researches for examining the disease process. Taken together, analysis by micro-CT makes it possible to quantify CIA lesions in rats when performed with pathological examination.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download