Abstract
The purpose of this study was to investigate the effects of porcine interleukin (IL)-2 and IL-4 genes on enhancing the immunogenicity of a porcine reproductive and respiratory syndrome virus ORF5 DNA vaccine in piglets. Eukaryotic expression plasmids pcDNA-ORF5, pcDNA-IL-2, and pcDNA-IL-4 were constructed and then expressed in Marc-145 cells. The effects of these genes were detected using an indirect immunofluorescent assay and reverse transcription polymerase chain reaction (RT-PCR). Characteristic fluorescence was observed at different times after pcDNA-ORF5 was expressed in the Marc-145 cells, and PCR products corresponding to ORF5, IL-2, and IL-4 genes were detected at 48 h. Based on these data, healthy piglets were injected intramuscularly with different combinations of the purified plasmids: pcDNA-ORF5 alone, pcDNA-ORF5 + pcDNA-IL-2, pcDNA-ORF5 + pcDNA-IL-4, and pcDNA-ORF5 + pcDNAIL-4 + pcDNA-IL-2. The ensuing humoral immune responses, percentages of CD4+ and CD8+ T lymphocytes, proliferation indices, and interferon-γ expression were analyzed. Results revealed that the piglets co-immunized with pcDNA-ORF5 + pcDNA-IL-4 + pcDNA-IL-2 plasmids developed significantly higher antibody titers and neutralizing antibody levels, had significantly increased levels of specific T lymphocyte proliferation, elevated percentages of CD4+ and CD8+ T lymphocytes, and significantly higher IFN-γ production than the other inoculated pigs (p < 0.05).
Porcine reproductive and respiratory syndrome (PRRS) caused by porcine reproductive and respiratory syndrome virus (PRRSV) is a highly contagious disease mainly characterized by premature delivery, miscarriage, stillbirth, mummified fetuses in pregnant sows, severe respiratory symptoms in sucking piglets, increased mortality before weaning, dyspnea and growth retardation in growing pigs, and decreased libido and semen quality in boars [3,7]. PRRS is one of the most severe viral diseases of swine that is widely spread throughout all pig-producing countries worldwide and has resulted in immense economic losses to the swine industry since the late 1980s [22]. PRRSV has become one of the most important swine pathogens in mainland China since the first outbreak in 1995. PRRSV is an enveloped virus with a positive-stand RNA genome approximately 15 kb in length. The genome contains a 5 untranslated region (5'-UTR), and genes (ORF1a and ORF1b) encoding the viral nonstructural proteins (Nsps) that are located at the downstream of the 5'-UTR. Additionally, genes (ORF1a, ORF1b, and ORF3 to ORF7) encoding viral structural proteins (GP2, E, GP3, GP4, GP5, M, and N) are located at the 3' end of the genome. An additional structural protein, GP5a, composed of 43~64 amino acids is encoded by an alternative ORF in the subgenomic viral mRNA encoding GP5, and is a novel structural protein recently discovered in all arteriviruses [11,17]. The genome also possesses a 3'-UTR tail and a poly (A) tail. Overall, ten open reading frames (ORFs) have been identified in the PRRSV genome.
PRRSV mainly invades porcine alveolar macrophages, thereby inducing immunosuppression and immunity failure of vaccines. Inactivated and attenuated PRRSV vaccines widely used in the swine industry have played an important role in PRRS prevention and cure. However, these vaccines are still associated with some defects such as poor immune protective effects and danger of toxic exposure [24] due to low immunogenicity and maternal antibody interference. Therefore, researchers in various countries are devoted to developing a new type of vaccine that is cheaper, safer, and more efficient. The recently developed DNA vaccine (also called gene engineering vaccine) has received much attention because it is not associated with a danger of virulence enhancement, is easy to prepare, has good stability, and is convenient to store and transport [13].
ORF5-encoded GP5 is the main structural protein and most important immunogenic protein of PRRSV. GP5 is a glycosylated envelope protein containing approximately 200 amino acids with an apparent molecular mass of 26 kDa. This protein can induce the production of virus-specific neutralizing antibodies and is characterize by high levels of immunogenicity. A study by Boroushan Pirzadeh and Dea [28] showed that DNA immunization with a plasmid encoding GP5 under the control of a human cytomegalovirus promoter can induce the generation of GP5-neutralizing antibodies in both pigs and BALB/c mice. A co-expressed eukaryotic expression plasmid constructed by Jiang et al. [15] using genes encoding modified PRRSV GP5 and M protein can elicit high specific cellular immune responses in immunized mice and weaned piglets. These studies demonstrated that the ORF5 gene is an important candidate gene for a PRRS DNA vaccine. Although the DNA vaccine has provided good experimental results, the associated immune efficacy is not as promising. Enhancement of the DNA vaccine efficacy has become a focus of researchers. The use of an immune adjuvant is important for improving the effects of the DNA vaccine. Cytokine adjuvant, co-stimulatory molecules adjuvant, and CpG DNA sequences are commonly used. A large number of studies have shown that cytokines as adjuvant can improve the immune response to DNA vaccines. It has been confirmed that IL-1, IL-2, IL-4, IL-18, and other cytokines are good adjuvants. IL-2 has been identified as one of the major cytokines responsible for regulating cell-mediated immune responses in mice [12]. Recent data showed that IL-2 may help stimulate cell-mediated immunity in swine [36]. IL-4 plays a major role in T-cell development and is thought to promote the differentiation of T helper cells into Th2 cells during an immune response [27]. Several studies in different animal models have also demonstrated that IL-4 can enhance antigen-specific humoral responses [18,39].
In the current study, a recombinant eukaryotic expression plasmid pcDNA-ORF5 was constructed. To explore the effect of cytokines IL-2 and IL-4 on pcDNA-ORF5 immunogenicity, eukaryotic expression plasmids pcDNA-ORF5, pcDNA-IL-2, and pcDNA-IL-4 were expressed in Marc-145 cells using Lipofectamine 2000 reagent (Invitrogen, USA). Healthy piglets were immunized using the eukaryotic expression plasmid pcDNA-ORF5 together with pcDNA-IL-2 and pcDNA-IL-4 as immunoadjuvants. The abilities of pcDNA-IL-2 and pcDNA-IL-4 to promote and enhance cellular immunity upon vaccination with a PRRSV-ORF5 DNA vaccine were comprehensively evaluated in order to provide a theoretical basis for further optimizing the clinical application of a PRRSV DNA vaccine.
PRRSV Guizhou isolate was stored in our laboratory [35]. For virus isolation, Marc-145 cells (Bioleaf Biotech, China) were first incubated with Dulbecco's modified Eagle's medium (DMEM; Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS; HyClone, USA), 100 units of penicillin(Solarbio, China), and 20 units of streptomycin (Solarbio) in a 37℃ humidified chamber (Thermo Fisher Sientific, USA) with 5% CO2. Next, the Marc-145 cells were infected with PRRSV Guizhou isolate. Briefly, a monolayer of Marc-145 cells were infected by the PRRSV Guizhou isolate for 1 h and then incubated in DMEM supplemented with 2% FBS. The infected cells were incubated in a 5% CO2 incubator at 37℃ and observed daily for 4 to 5 days for cytopathic effects (CPEs) under a microscope (Olympus, Japan).
When approximately 80% of the cells showed CPEs, the cells were freeze-thawed three times, cellular debris was removed by low-speed centrifugation, and the supernatant fluid was stored at -70℃ for the following experiments. Viral RNA was extracted from the supernatant fluid using a QIAamp Viral RNA Mini Kit (Qiagen, Germany) according to the manufacturer's protocol. To obtain the complete ORF5 gene of the PRRSV Guizhou strain, one pair of specific primers was designed using Primer Premier 5.0 software (PREMIER Biosoft, Canada) according to the ORF5 gene sequence of PRRSV Guizhou-1 strain (GenBank No. EU259060), CH-1a strain (GenBank No. AY032626), and VR-2332 strain (GenBank No. PRU87392) published in GenBank. The forward primer sequence was 5'-CGGGATCCATGTTGGGGAAGTGCT-3' (containing a BamHI site in the 5' terminal) and the reverse primer sequence was 5'-CGGAATTCCTAGAGACGACCCCATT-3' (containing an EcoRI site in 5' terminal). The extracted total viral RNA was used as a template for cDNA synthesis. Reverse transcription (RT) reaction system was: 10 µL of PCR reaction mixture consists with 5 µL total viral RNA, 2 µL 5× SuperscriptIIIBuffer, 0.5 µL 0.1 M DTT, 0.5 µL 10 M dNTPs, 0.25 µL RNase-Inhibitor, 0.5 µL SuperscriptIII reverse transcriptase (Invitrogen), 1.25 µL 10 M downstream primer, react at 42℃ for 1 h, then get the cDNA template.
The cDNA was subsequently used for PCR amplification with an LA PCR Kit Ver.2.1 (Takara Bio, China) according to the manufacturer's protocol. The PCR reaction system as follows: 10× LA Taq Buffer 5 µL (Takara Bio), 2.5 mM dNTP Mixture 4 µL (Takara Bio), cDNA 2 µL, 2 µL each of forward and reverse primer (20 pmol/µL), LA Taq polymerase 1 µL (5 U/µL) (Takara Bio), adding ddH2O to 50 µL in total. The reaction conditions were: denaturation for 5 min at 94℃ then 35 cycles of denaturation at 94℃ for 30 sec, annealing at 58℃ for 30 sec, and extension at 72℃ for 90 sec followed by a final extension step at 72℃ for 10 min. The amplicons were separated by electrophoresis in a 1% agarose gel and the gene fragments were recoverd with a Gel Extraction Kit (Qiagen, Germany) according to the manufacturer's protocol. The recovered target gene product was ligated into a pMD19-T vector (Takara Bio) and used to transformed competent E. coli Top10 cells (Promega, USA). Positive transformants were selected by colony PCR with the above mentioned primers and restriction enzyme digestion of recombinant plasmid with BamHI and EcoRI (Takara Bio). The recombinant positive clones were sent to TaKaRa Biotechnology for sequencing in both directions at least twice to avoid artifacts.
The pMD19-T-ORF5 recombinant plasmid and pcDNA3.1(+) vector (Invitrogen) were double digested separately with BamHI and EcoRI (Takara Bio). The digested ORF5 gene and pcDNA3.1(+) fragments were gel-purified using a QIAquick Gel Extraction Kit (Qiagen) and ligated with T4 DNA ligase (Takara Bio). The ligation products were used to transform E. coli Top10 competent cells. Positive clones were screened and identified by colony PCR as described above. The recombinant plasmid was named pcDNA-ORF5. Eukaryotic expression plasmids pcDNA-IL-2 and pcDNA-IL-4 were constructed as previously described [32,33].
The E. coli Top10 cells which contained the recombinant plasmid pcDNA3.1(+)-ORF5 were used to inoculate 5 mL 2×YT liquid medium (Takara Bio, Japan) containing 50 µg/mL ampicillin, and incubated with shaking at 0.80 × g (120 rpm, rotational radius is 5 cm) at 37℃ for 12~16 h. Plasmid DNA was extracted with an E.Z.N.A. Plasmid Mini Kit (Takara Bio, Japan), and the DNA concentration was determined with a spectrophotometer (Thermo Fisher Sientific) at 260 nm. The small-scale preparation of pcDNA-IL-2 and pcDNA-IL-4 was conducted using the same protocol.
Marc-145 cells were transfected with the three eukaryotic expression plasmids pcDNA-ORF5, pcDNA-IL-2, and pcDNA-IL-4 using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. At the same time, the pcDNA3.1(+) plasmid was used as the control. After incubation at 37℃ for 4 h, the cell culture supernatant was aspirated and replaced with DMEM supplemented with 2% FBS, then the transfected cells were incubated in a 5% CO2 incubator at 37℃. Expression of pcDNA-ORF5 in the Marc-145 cells was analyzed by an indirect immunofluorescence assay (IFA) as follows. The cells were collected at 24, 48, and 67 h posttransfection and fixed in a cold methanol: acetone (1:1) solution at -20℃ for 15 min. The fixed cells were washed with phosphate-buffered saline (PBS) and incubated with swine anti-PRRSV hyperimmune sera at a 1 : 100 dilution (prepared by the Animal Disease Lab, China) for 2 h at room temperature, followed by incubation for 1 h at room temperature with fluorescein isothiocyanateconjugated goat anti-porcine IgG at a 1 : 100 dilution (Solarbio). Cells expressing the control pcDNA3.1(+) as a reference were treated in the same manner. The cells were observed and photographed under an inverted fluorescence microscope (Nikon, Japan). Additionally, total RNAs from the Marc-145 cells transfected with plasmids pcDNA-ORF5, pcDNA-IL-2, pcDNA-IL-4 and pcDNA3.1(+) were extracted from 200 µL of the infected supernatants at 48 h using a QIAamp Viral RNA Mini Kit (Qiagen) according to the manufacturer's instructions, respectively. Reverse transcription PCR was conducted to monitor plasmid expression, the primers used to amply porcine IL-2 and IL-4 genes as previously described [32,33]. The protocols were the same as above.
Cells transfected with pcDNA-ORF5 were collected using cell scraper (Corning, USA) at 48 h post-transfection. The cells were washed twice with PBS, incubated in lysis buffer (Qiagen), and boiled for 10 min, and then the lysates were centrifuged for 5 min at 12,000 × g at 4℃. After centrifugation, proteins in the lysis were subjected to SDS-PAGE and Western blotting. After separation by SDS-PAGE, the proteins were transferred onto nitrocellulose membranes (Solarbio) using a semi-wet transfer cell (Bio-Rad, USA). The membranes were blocked with 5% skimmed milk (Boster, China) in TBST [50 mM Tris-HCl, 150 mM NaCl, and 0.05% Tween 20 (pH 7.5)] for 1 h at room temperature. The blocked membranes were then incubated with swine anti-PRRSV hyperimmune sera at a dilution of 1 : 100 in TBST at 37℃ for 1 h, washed four times for 5 min each in PBS, and subsequently incubated with horseradish peroxidase (HRP)-conjugated goat anti-porcine IgG (Solarbio) at a dilution of 1 : 1000 in TBST for 2~3 h at 37℃. After the membranes were washed in TBST four times for 5 min each, 3,3'-diaminobenzidine (DAB; Zhongshan Golden Bridge Biotech, China) was added to detect antibody binding. SDS-PAGE analysis and Western blotting for pcDNA-IL-2 and pcDNA-IL-4 expression were conducted as described above except for the use of different primary and secondary antibodies. For Western blotting of pcDNA-IL-2 and pcDNA-IL-4, porcine anti-IL-2 and anti-IL4 monoclonal antibodies (Boster) were used as primary antibodies at a dilution of 1 : 100 in TBST. HRP-conjugated goat anti-mouse IgG (Solarbio) was used as the secondary antibody at a dilution of 1 : 2000 in TBST.
Large-scale preparation of pcDNA-ORF5, pcDNA-IL-2, and pcDNA-IL-4 plasmids was performed using an EndoFree Plasmid ezFilter Mega 10 Kit (Biomiga, USA) according to the manufacturer's protocol. The plasmid concentrations were measured with a spectrophotometer at 260 nm. The plasmids were stored at -20℃ until use.
Twenty-four 1-month-old crossbreed (Landrace local stock) PRRSV-free piglets provided by the pig farm of Guizhou University were tested by enzyme-linked immuno sorbent assay (ELISA) were randomly divided into six groups that were housed separately. All the animals were housed and used for experiments according to the guidelines established by Guizhou University for the ethical use of animals in research. Group 1, 2, 3 ,4 and 5 were inoculated intramuscularly twice with 1 mL of PBS containing 800 µg of pcDNA3.1(+) (as negative control), 800 µg of pcDNA-ORF5, 800 µg of pcDNA-ORF5 + 400 µg of pcDNA-IL-2,800 µg of pcDNA-ORF5 + 400 µg of pcDNA-IL-4, 800 µg of pcDNA-ORF5 + 400 µg of pcDNA-IL-2 + 400 µg of pcDNA-IL-4, respectively. Meanwhile, group 6 was vaccinated in the same way with inactivated PRRSV vaccine (China Animal Husbandry Industry, China) at a dose of 2 mL each piglet according to the manufacturer's protocol. All the piglets were inoculated intramuscularly twice on days 0 and 14 (Table 1).
Sera were collected from all piglets in each group at 0, 7, 14, 28, and 35 days post-immunization (dpi) to detect circulating and neutralizing antibodies against PRRSV. Moreover, at 35 days blood samples were taken for a lymphocyte proliferation assay, analysis of CD4+ and CD8+ T lymphocyte percentages, and an IFN-γ response assay.
Swine blood samples were collected from each pig in all groups at 0, 7, 14, 28, and 35 dpi, then centrifuge for 5 min at 5,000 × g at room temperature to separate the serum, to identify the presence of specific anti-PRRSV IgG antibodies by ELISA using a PRRSV Antibody (PRRS-Ab) Test Kit (LSI, France) according to the manufacturer's protocol. This ELISA uses membrane proteins (M; GP2, GP3, GP4, and GP5) of PRRSV as the major envelope antigen, and can therefore detect GP5-specific antibodies.
Serum samples collected from the pigs were heat inactivated for 45 min at 56℃, diluted in 2-fold serial dilutions to 2-5 times, and then incubated for 1 h at 37℃ in the presence of PRRSV Guizhou isolate 100 tissue culture infective doses (TCID50). The mixture was transferred to Marc-145 cells monolayers that had been seeded in 96-well microtitration plates (Corning, USA) 48~72 h earlier and incubated at 37℃ in 5% CO2 for 96 h. Cells in the plates were examined for CPEs after inoculation 48 h. Neutralization titers were expressed as log2 of the reciprocal of the highest serum dilution that completely inhibited viral replication in 50% of the wells.
A 3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium-bromide (MTT) assay was used to evaluate T lymphocyte proliferation. Briefly, lymphocytes were isolated from heparinized swine blood collected 35 dpi by Ficoll-Hypaque density gradient centrifugation for 20 min at 5,000 × g at 4℃, washed three times with modified Hanks Balanced Salt Mixture (HBSS; Gibco, USA), and seeded at 5 × 105 cells/well into 96-well U-bottom culture plates (Nunc, Denmark) in complete Roswell Park Memorial Institute (RPMI) medium 1640 (Gibco) with 10% FBS. Each sample was plated and assayed in triplicate. The T lymphocytes were stimulated with the field isolate PRRSV Guizhou strain at a multiplicity of infection (MOI) of 1 as the specific antigen. Cell lysate extracted from mock-infected Marc-145 cells with PBS was used as a negative control, and concanavalin A (Con A, 5 µg/mL; Sigma, USA) served as a positive control. After incubation at 37℃ for 45 h in 5% CO2 and subsequent incubation for 4 h with MTT of 0.05% concentration (Sigma), proliferation was detected as previously described [30]. The plates were read at 570 nm with a microplate reader (Thermo Fisher Scientific). The stimulation index (SI) was calculated as the ratio of the average optical density (OD) of wells containing antigen-stimulated cells to the average OD of wells containing cells cultured with medium alone.
At 35 dpi, peripheral blood samples were collected from each piglet. Next, 100 µL of the cells (2 × 105 cells) were stained for 30 min with fluorescein isothiocyanate (FITC)-labeled mouse anti-porcine CD4a antibody (0.2 mg/mL; Southern Biotechnology Associates, USA) at 4℃. Another 100 µL of lympholyte sample (2 × 105 cells) were stained for 30 min with phycoerythrin (PE) labeled mouse anti-porcine CD8a antibody (0.2 mg/mL; Southern Biotechnology Associates) at 4℃. After washing, the cells were analyzed with a FC500 flow cytometer (Beckman Coulter, USA), and the percentages of CD4+ and CD8+ T lymphocytes were calculated.
To further evaluate cellular immune responses, IFN-γ levels in peripheral blood isolated from the vaccinated piglets at 35 dpi were measured using a commercial ELISA kit (Pig Interferon-γ, IFN-γ ELISA Kit; CUSABIO, USA) according to the manufacturer's instructions.
All data are presented as the mean (X) ± standard deviation (SD). Differences in humoral or cellular immune responses between the various groups were identified by an analysis of variance and t test. The p values < 0.05 were considered statistically significant. All statistical analyses were performed with SPSS software (ver. 17.0; SPSS, USA).
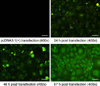
Double-digestion identification and sequence analysis showed that recombinant eukaryotic expression plasmid pcDNA-ORF5 was successfully constructed. To determine whether the foreign gene could be exogenously expressed, Marc-145 cells were transfected with recombinant plasmids and the resulting fluorescence was detected. As shown in Fig. 1, obvious green fluorescence was detected in the cells transfected with pcDNA-ORF5. Twenty-four h after the Marc-145 cells were transfected with the recombinant plasmids, fluorescence was observed. Sixty-seven h after the Marc-145 cells were transfected with the recombinant plasmids; green fluorescence almost reached a maximum intensity. Fluorescence was found in roughly 92% of the cells, indicating that the recombinant eukaryotic expression plasmid pcDNA-ORF5 could be easily expressed in vitro. Moreover, mRNA was extracted from the transfected Marc-145 cells and plasmid expression was verified by RT-PCR. As shown in Fig. 2, positive bands corresponding to IL-2, IL-4, and ORF5 were observed with sizes of approximately 526 bp, 411 bp, and 603 bp, respectively, as predicted. No band was found for the Marc-145 cells transfected with pcDNA3.1(+), confirming that the plasmids were expressed in Marc-145 cells.
The cells transfected with pcDNA-ORF5, pcDNA-IL-2, and pcDNA-IL-4 were lysed for SDS-PAGE and Western blotting analysis. As shown in Fig. 4, bands 23 kDa (Fig. 3A), 18 kDa (Fig. 3B), and 15 kDa (Fig. 3C) in size were detected by Western blotting. The sizes of all bands were as predicted, proving that the foreign genes were truly expressed in the mammalian cells.
Immunogenicity of the recombinant DNA vaccines was evaluated in piglets. As shown in Fig. 4, specific anti-PRRSV IgG antibodies in pigs from the different groups were detected with an ELISA using pcDNA3.1(+) as the negative control. The results showed that all inoculated pigs except for those in the control group produced high levels of IgG antibodies specific for PRRSV. The antibody levels were significantly higher than those of the control group (p < 0.05). After 28 and 35 days post-inoculation, the antibody levels in pcDNA-ORF5 + pcDNA-IL-2 + pcDNA-IL-4 immune group was significantly higher than those in other five groups (p < 0.05). At 35 days, the antibody levels in both groups 3 and 4 were significantly higher than those in groups 2 and 6 (p < 0.05).
Sera from the inoculated pigs were tested for the ability to neutralize PRRSV in Marc-145 cells. As shown in Table 2, the titers of neutralizing antibody from group 5 pigs ranged from 1 : 8.02 to 1 : 19.28, and were significantly higher than those observed for the other groups (p < 0.05). These results indicated that IL-2 and IL-4 can enhance the immunogenicity of the PRRSV ORF5 gene vaccine. The titers of neutralizing antibody from groups 3 and 4 were higher than those of groups 2 and 6. No significant difference (p > 0.05) was observed at 28 dpi, but this was not the case at 42 dpi (p < 0.05). No serum neutralizing antibody activity was observed for the negative control group throughout the assay.
As shown in Fig. 5, all five vaccinated groups (groups 2~6) developed specific T lymphocyte proliferation responses, and the levels of T lymphocyte proliferation in these groups were significantly higher than those of the pcDNA3.1(+)-inoculated group (p < 0.05). Group 5 had significantly greater specific T lymphocyte proliferation responses than any other vaccinated groups (p < 0.05). Furthermore, the levels of T lymphocyte proliferation in groups 3 and 4 were significantly higher than those of groups 2 and 6 (p < 0.05).
A significant increase (p < 0.05) in the absolute numbers of both CD4+ and CD8+ T cells was found in other five groups compared to group 1 (Table 3). The percentages of CD4+ and CD8+ T lymphocytes in group 5 were consistently the highest out of all vaccinated groups (p < 0.05). Moreover, groups 3 and 4 had higher CD4+ and CD8+ T lymphocyte percentages than groups 2 and 6, but this difference was not significant (p > 0.05).
The pigs immunized with DNA and PRRSV vaccines (Fig. 6) produced significantly higher levels (p < 0.05) of IFN-γ than the animals treated with pcDNA3.1(+). Group 5 generated significantly higher IFN-γ levels than any other group (p < 0.05). These results indicated that groups 3, 4, and 5 mounted a Th1-like cellular immune response and group 5 produced a stronger cellular immune response.
Currently, no specific treatment is available for PRRS. Therefore, vaccination is an efficient strategy for controlling this disease. GP5 encoded by the PRRSV ORF5 gene is a major glycosylation envelope protein and a primary target of neutralizing antibody. Studies have shown that the GP5 protein may be involved in the process of virus particle binding receptor identify PRRSV [8,31]. Moreover, previous reports have shown that GP5 induces relatively high titers of neutralizing antibodies and provides partial protection against PRRSV [6,16,26]. However, Li and Murtaugh [19] recently reported that the GP5/M ectodomain peptide epitopes are accessible for host antibody recognition but are not associated with antibody-mediated virus neutralization. In their study, the GP5/M ectodomain peptide epitopes were expressed with myc and his tags in BL21 (DE3)-RP cells, thereby producing a prokaryotic expression product instead of a eukaryotic expression product. Ivan Díaz et al. [10] demonstrated that immunization with a DNA vaccine containing PRRSV ORF5 could induce PRRSV-specific IFN-γ-secreting cells. This enabled faster production of anti-PRRSV IgG antibody and significantly reduced viremia following virulent virus challenge. However, immunization with the DNA vaccine failed to elicit the generation of neutralizing antibodies and may have promoted the exacerbation of clinical disease after experimental viral challenge. In this investigation, one group was injected three times with ORF7, the second group was immunized with three inoculations of plasmids containing ORF5 and ORF6, and the third group was immunized with pcDNA3.1(+) as the control. The purpose of the study was to explore whether ORF6 has a negative effect on ORF5. However, none of the pigs were immunized with ORF5 alone.
Previous reports have also demonstrated that PRRSV has antibody-dependent enhancement (ADE) capabilities [14,35]. Other studies also showed that the immunodominant and non-neutralizing epitope comprised of (A/V) 27 L (Leucine) V (Valine) N (Asparagine) near the primary neutralizing epitope (PNE) may act as a decoy, thus eliciting the production of most antibodies against GP5 and delaying the induction of neutralizing antibodies for at least 3 weeks. GP5 glycosylation sites are concentrated in front of N44 sugar chains, and neutralizing epitopes are located exactly between the N44 sugar chain and a preceding sugar chain [26]. The effective ability of neutralizing antibodies to identify specific neutralizing epitopes is reduced due to steric hindrance that allows the virus to evade recognition by the antibody [1]. Thus, current studies have implied that the use of viral proteins for genetically engineered vaccines might be a double-edged sword, and a better understanding of the use of engineered vaccines and immunopathogenesis of PRRS is necessary.
Presently, there are two major categories of commercially available PRRSV vaccines: a PRRSV inactivated vaccine and modified-live virus vaccine. The former only provides partial immunity to the highly pathogenic Chinese PRRSV [40]. Although the latter confers solid protection against clinical disease induced by homologous infection, virulence of the virus may return [24,25] and restrict the application of this vaccination approach. Therefore, it is necessary to improve PRRSV vaccines or produce new generation vaccines against PRRSV. IL-2, IL-4, IFN-γ, IL-7, granulocyte-macrophage colony stimulating factor (GM-CSF), and IL-18 have been previously found to have potent adjuvant activity [30,5, 29,34,37]. Among these factor, IL-2 and IL-4 are pleiotropic cytokines and have been widely used as adjuvants to enhance immune responses of many vaccine antigens. Zhang et al. [38] reported that the expression of recombinant IL-2 protein can significantly improve the antibody titers of foot-and-mouth disease vaccines in sows and bred sows. Chen et al. [4] constructed a fusion plasmid capable of expressing classical swine fever virus (CSFV) E2 and IL-2 genes, and transfected BHK-21 cells. The results demonstrated that active E2 and IL-2 proteins could be expressed in vitro, and the simultaneous expression of IL-2 and CSFV protective antigenic genes could increase the immune response in mice. Another investigation by Zhang et al. [39] showed that IL-4 can promote B cell proliferation and differentiation, indirectly increase the effects of killer T cells against cysticerci, augment the production of anti-cysticercosis antibodies, increase antigen presentation, and promote the secretion of cytokines involved in immune regulation.
In the present study, eukaryotic expression plasmids pcDNA-ORF5, pcDNA-IL-2, and pcDNA-IL-4 were produced. Using these constructs, we comparatively tested the effects of swine IL-2 and IL-4 on the modulation of DNA vaccine-induced immune responses in piglets. Neutralizing antibody is a major component of humoral immunity that provides protection against PRRSV infection and plays an important role in the prevention or reduction of viral spread among pigs [21]. At 35 days, both the IgG antibody levels and neutralizing antibody titers from piglets co-immunized with pcDNA-ORF5 + pcDNA-IL-2 or pcDNA-ORF5 + pcDNA-IL-4 were significantly higher than those in animals immunized with the pcDNA-ORF5 and PRRSV inactivated vaccines. These data demonstrated that both IL-2 and IL-4 can enhance immunogenicity of the PRRSV ORF5 gene vaccine. Twenty-eight and 35 days after inoculation, the levels of IgG antibody and neutralizing antibody titers in piglets co-immunized with pcDNA-ORF5 + pcDNA-IL-2 + pcDNA-IL-4 were significantly higher than those found in the other vaccinated groups (p < 0.05), indicating that IL-2 and IL-4 have a synergistic effect on the generation of serum neutralizing antibody titers.
Cell-mediated immunity plays an important role against PRRSV infection, and it has been found that T cell-mediated immunity is necessary for effective protection against PRRSV [23]. Early studies showed that pigs recovering from experimental PRRSV infection have strong lymphocyte proliferative responses [2,20]. Moreover, the specific cytokines response (mainly an IFN-γ response) produced during infection is a determinant for inducing an effective cellular immune response [9]. CD4+ lymphocytes are a type of helper T (Th) cell that play an important role in the immune response. CD8+ lymphocytes are a type of cytotoxic T cell, also known as cytotoxic T lymphocytes (CTLs), that specifically secret various cytokines and are involved in immune responses. The percentages of CD4+ and CD8+ lymphocyte subpopulations are another important measure for evaluating the efficacy of DNA vaccines. In the current study, T lymphocyte proliferation, percentages of CD4+ and CD8+ T lymphocytes, and IFN-γ production were comparatively measured to evaluate the DNA vaccine-induced cellular immune responses. The experimental results showed that the piglets co-immunized with pcDNA-ORF5 + pcDNA-IL-2, pcDNA-ORF5 + pcDNA-IL-4, or pcDNA-ORF5 + pcDNA-IL-4 + pcDNA-ORF5 had significant specific T lymphocyte proliferation responses, increased numbers of CD4+ and CD8+ T lymphocytes, and higher levels of IFN-γ production than animals receiving the pcDNA-ORF5 vaccine or PRRSV inactivated vaccine (p < 0.05). These findings indicated that the DNA vaccine resulted in cellular immunity. In addition, the study findings showed that the pcDNA-ORF5 + pcDNA-IL-2 vaccine had a better effect on piglets than the pcDNA-ORF5 + pcDNA-IL-4 vaccine. These results suggest that swine IL-4 may down-regulate or inhibit the induction of the cellular response induced by a vaccine or virus infection. We speculate that this effect may be because IL-2 is mainly involved in cell-mediated immune responses whereas IL-4 usually affects B cell activation, regulates the production of antibodies, and inhibits T cells expressing the IL-2 receptor. Our results indicated that the pcDNA-ORF5 co-delivered with pcDNA-IL-4 or pcDNA-IL-2 could have significantly promoted the activation of vaccine-induced virus-specific cellular immune responses in pigs.
In conclusion, the recombinant plasmid pcDNA-ORF5 elicited cellular and specific immune responses in piglets although these effects were not as potent as ones produced by the PRRSV inactivated vaccine. Compared to the expression of GP5 alone or the PRRSV inactivated vaccine, the recombinant plasmids pcDNA-IL-2 and pcDNA-IL-4 exerted significant immunoenhancement effects when administered with pcDNA-ORF5. Developing an effective immune adjuvant is an important concern in vaccine research. Although several works have been performed to explore the nature and immune response characteristics of the ORF5 protein, many problems must be overcome before an effective vaccine can be developed.
Figures and Tables
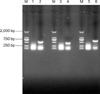 | Fig. 2RT-PCR results for the transfected cells. M, DL2000 DNA marker; Lanes 1, 3, and 5, RT-PCR products from cells transfected with pcDNA3.1(+); Lane 2, RT-PCR product from cells transfected with pcDNA-IL-2; Lane 4, RT-PCR product from cells transfected with pcDNA-IL-4; Lane 6, RT-PCR product from cells transfected with pcDNA-ORF5. |
 | Fig. 3Detection of recombinant proteins encoded by expression plasmids pcDNA-ORF5(A), pcDNA-IL-2 (B), and pcDNA-IL-4 (C) using Western blotting. |
 | Fig. 4Humoral immune responses in vaccinated piglets from the different groups detected with a commercial ELISA kit. |
Acknowledgments
This work was supported by the Guiyang Municipal Science and Technology Fund [No. (2005)4-11], Science and Technology Fund of Guizhou province [No. (2013)2110], and Agricultural Research Project of Guizhou province [No. (2011)3103].
References
1. An T, Zhou Y, Liu G, Tian Z, Li J, Qiu H, Tong G. Genetic diversity and phylogenetic analysis of glycoprotein 5 of PRRSV isolates in mainland China from 1996 to 2006: coexistence of two NA-subgenotypes with great diversity. Vet Microbiol. 2007; 123:43–52.

2. Bautista EM, Molitor TW. Cell-mediated immunity to porcine reproductive and respiratory syndrome virus in swine. Viral Immunol. 1997; 10:83–94.

3. Beyer J, Fichtner D, Schirrmeier H, Polster U, Weiland E, Wege H. Porcine reproductive and respiratory syndrome virus (PRRSV): kinetics of infection in lymphatic organs and lung. J Vet Med B Infect Dis Vet Public Health. 2000; 47:9–25.

4. Chen CF, Yu XL, Ma ZH, Li ZS, Li HW, Tu CC, Yin Z. Studies on construction and immunity enhancer IL-2 and IL-3 of the double expression plasmid of classical swine fever virus E2. Zhongguo Nong Ye Ke Xue. 2002; 11:1406–1410.
5. Chen H, Zhong F, Li X, Wang L, Sun Y, Neng C, Zhang K, Li W, Wen J. Effects of canine IL-2 and IL-7 genes on enhancing immunogenicity of canine parvovirus VP2 gene vaccine in mice. Wei Sheng Wu Xue Bao. 2012; 52:1392–1399.
6. Chia MY, Hsiao SH, Chan HT, Do YY, Huang PL, Chang HW, Tsai YC, Lin CM, Pang VF, Jeng CR. The immunogenicity of DNA constructs co-expressing GP5 and M proteins of porcine reproductive and respiratory syndrome virus conjugated by GPGP linker in pigs. Vet Microbiol. 2010; 146:189–199.

7. Chung WB, Lin MW, Chang WF, Hsu M, Yang PC. Persistence of porcine reproductive and respiratory syndrome virus in intensive farrow-to-finish pig herds. Can J Vet Res. 1997; 61:292–298.
8. Delputte PL, Vanderheijden N, Nauwynck HJ, Pensaert MB. Involvement of the matrix protein in attachment of porcine reproductive and respiratory syndrome virus to a heparinlike receptor on porcine alveolar macrophage. J Virol. 2002; 76:4312–4320.

9. Diaz I, Darwich L, Pappaterra G, Pujols J, Mateu E. Different European-type vaccines against porcine reproductive and respiratory syndrome virus have different immunological properties and confer different protection to pigs. Virology. 2006; 351:249–259.

10. Díaz I, Ganges L, Galindo-Cardiel I, Tarradas J, Álvarez B, Lorca-Oró C, Pujols J, Gimeno M, Darwich L, Domingo M, Domínguez J, Mateu E. Immunization with DNA vaccines containing porcine reproductive and respiratory syndrome virus open reading frames 5, 6, and 7 may be related to the exacerbation of clinical disease after an experimental challenge. Viral Immunol. 2013; 26:93–101.

11. Firth AE, Zevenhoven-Dobbe JC, Wills NM, Go YY, Balasuriya UB, Atkins JF, Snijder EJ, Posthuma CC. Discovery of a small arterivirus gene that overlaps the GP5 coding sequence and is important for virus production. J Gen Virol. 2011; 92:1097–1106.

12. Gaffen SL, Liu KD. Overview of interleukin-2 function, production and clinical applications. Cytokine. 2004; 28:109–123.

13. Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol. 2000; 18:927–974.

14. Huisman W, Martina BEE, Rimmelzwaan GF, Gruters RA, Osterhaus ADME. Vaccine-induced enhancement of viral infections. Vaccine. 2009; 27:505–512.

15. Jiang Y, Fang L, Xiao S, Li B, Pan Y, Luo R, Chen H. A suicidal DNA vaccine co-expressing two major membrane-associated proteins of porcine reproductive and respiratory syndrome virus antigens induce protective responses. Biotechnol Lett. 2009; 31:509–518.

16. Jiang Y, Xiao S, Fang L, Yu X, Song Y, Niu C, Chen H. DNA vaccines co-expressing GP5 and M proteins of porcine reproductive and respiratory syndrome virus (PRRSV) display enhanced immunogenicity. Vaccine. 2006; 24:2869–2879.

17. Johnson CR, Griggs TF, Gnanandarajah J, Murtaugh MP. Novel structural protein in porcine reproductive and respiratory syndrome virus encoded by an alternative ORF5 present in all arteriviruses. J Gen Virol. 2011; 92:1107–1116.

18. Kim JJ, Yang JS, Manson KH, Weiner DB. Modulation of antigen-specific cellular immune responses to DNA vaccination in rhesus macaques through the use of IL-2, IFN-gamma, or IL-4 gene adjuvants. Vaccine. 2001; 19:2496–2505.

19. Li J, Murtaugh MP. Dissociation of porcine reproductive and respiratory syndrome virus neutralization from antibodies specifito major envelope protein surface epitopes. Virology. 2012; 433:367–376.

20. López Fuertes L, Doménech N, Alvarez B, Ezquerra A, Domínguez J, Castro JM, Alonso F. Analysis of cellular immune response in pigs recovered from porcine respiratory and reproductive syndrome infection. Virus Res. 1999; 64:33–42.

21. Lopez OJ, Oliveira MF, Garcia EA, Kwon BJ, Doster A, Osorio FA. Protection against porcine reproductive and respiratory syndrome virus (PRRSV) infection through passive transfer of PRRSV-neutralizing antibodies is dose dependent. Clin Vaccine Immunol. 2007; 14:269–275.

22. Lunney JK, Benfield D, Rowland RR. Porcine reproductive and respiratory syndrome virus: an update on an emerging and re-emerging viral disease of swine. Virus Res. 2010; 154:1–6.

23. Martelli P, Gozio S, Ferrari L, Rosina S, De Angelis E, Quintavalla C, Bottarelli E, Borghetti P. Efficacy of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine in pigs naturally exposed to a heterologous European (Italian cluster) field strain: clinical protection and cell-mediated immunity. Vaccine. 2009; 27:3788–3799.

24. Meng XJ. Heterogenecity of porcine reproductive and respiratory syndrome virus: implications for current vaccine efficacy and future vaccine development. Vet Microbiol. 2000; 74:309–329.

25. Opriessnig T, Halbur PG, Yoon KJ, Pogranichniy RM, Harmon KM, Evans R, Key KF, Pallares FJ, Thomas P, Meng XJ. Comparison of molecular and biological characteristics of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine (ingelvac PRRS MLV), the parent strain of the vaccine (ATCC VR2332), ATCC VR2385, and two recent field isolates of PRRSV. J Virol. 2002; 76:11837–11844.

26. Ostrowski M, Galeota JA, Jar AM, Platt KB, Osorio FA, Lopez OJ. Identification of neutralizing and nonneutralizing epitopes in the porcine reproductive and respiratory syndrome virus GP5 ectodomain. J Virol. 2002; 76:4241–4250.

28. Pirzadeh B, Dea S. Immune response in pigs vaccinated with plasmid DNA encoding ORF5 of porcine reproductive and respiratory syndrome virus. J Gen Virol. 1998; 79:989–999.

29. Rompato G, Ling E, Chen Z, Van Kruiningen H, Garmendia AE. Positive inductive effect of IL-2 on virus-specific cellular responses elicited by a PRRSV-ORF7 DNA vaccine in swine. Vet Immunol Immunopathol. 2006; 109:151–160.

30. Shen G, Jin N, Ma M, Jin K, Zheng M, Zhuang T, Lu H, Zhu G, Jin H, Jin M, Huo X, Qin X, Yin R, Li C, Li H, Li Y, Han Z, Chen Y, Jin M. Immune responses of pigs inoculated with a recombinant fowlpox virus coexpressing GP5/GP3 of porcine reproductive and respiratory syndrome virus and swine IL-18. Vaccine. 2007; 25:4193–4202.

31. Sur JH, Doster AR, Osorio FA. Apoptosis induced in vivo during acute infection by porcine reproductive and respiratory syndrome virus. Vet Pathol. 1998; 35:506–514.

32. Wang B, Tang DY, Li CY, Zeng ZY, Huang T, Xu J. Cloning and eukaryotic expression vector construction of IL-2 gene of Landrace. Heilongjiang Xu Mu Shou Yi. 2010; 40:40–43.
33. Wang B, Tang DY, Li CY, Zeng ZY, Wang F, Gan ZL. Cloning and eukaryotic expression vector construction of IL-4 gene of Landrace. Zhu Ye Ke Xue. 2010; 27:80–82.
34. Wang X, Li J, Jiang P, Li Y, Zeshan B, Cao J, Wang X. GM-CSF fused with GP3 and GP5 of porcine reproductive and respiratory syndrome virus increased the immune responses and protective efficacy against virulent PRRSV challenge. Virus Res. 2009; 143:24–32.

35. Wang ZR. Isolation and identification of Guizhou strain of porcine peproduetive and pespiratory syndrome virus and construction of the eukaryotic expression plasmid of ORF5 gene. Guiyang: Guizhou University;2008. MA thesis.
36. Wong HT, Cheng SCS, Sin FWY, Chan EWC, Sheng ZT, Xie Y. A DNA vaccine against foot-and-mouth disease elicits an immune response in swine which is enhanced by co-administration with interleukin-2. Vaccine. 2002; 20:2641–2647.

37. Zhang G, Zhao X, Hu Y, Li Z, Yiao Y. Effects of recombinant IL-2 on the immunization against Mouth and Foot Disease Virus in pigs. Henan Xu Mu Shou Yi. 2002; 23:4–5.
38. Zhang W, Wu S, Liu P, Li Y, Wang L. Study on the level of IL-4, IL-5 and IL-10 of cysticercosis patients. Zhong Guo Di Fang Bing Xue Za Zhi. 2005; 24:437–438.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download