Abstract
Feline leukemia virus (FeLV) causes a range of neoplastic and degenerative diseases in cats. To obtain a more sensitive and convenient diagnosis of the disease, we prepared monoclonal antibodies specific for the FeLV p27 to develop a rapid diagnostic test with enhanced sensitivity and specificity. Among these antibodies, we identified two clones (hybridomas 8F8B5 and 8G7D1) that specifically bound to FeLV and were very suitable for a diagnostic kit. The affinity constants for 8F8B5 and 8G7D1 were 0.35 × 109 and 0.86 × 109, respectively. To investigate the diagnostic abilities of the rapid kit using these antibodies, we performed several clinical studies. Assessment of analytical sensitivity revealed that the detection threshold of the rapid diagnostic test was 2 ng/mL for recombinant p27 and 12.5 × 104 IU/mL for FeLV. When evaluating 252 cat sera samples, the kit was found to have a kappa value of 0.88 compared to polymerase chain reaction (PCR), indicating a significant correlation between data from the rapid diagnostic test and PCR. Sensitivity and specificity of the kit were 95.2% (20/21) and 98.5% (257/261), respectively. Our results demonstrated that the rapid diagnostic test would be a suitable diagnostic tool for the rapid detection of FeLV infection in cats.
Feline leukemia virus (FeLV) is one of the most important infectious pathogens that causes death in cats and is broadly spread worldwide [12,17,24]. FeLV was discovered among cats that lived in a cluster-household where several animals had developed lymphosarcoma [19]. The virus is an enveloped, positive single-stranded RNA retrovirus. Cats are usually infected by direct contact with infected cats [12,31,33], mostly via oronasal exposure to saliva and nasal secretions containing high levels of the virus especially through mutual grooming and sharing food dishes or water bowls [11,12,17]. Vertical transmission occasionally occurs [35] but is of secondary importance. FeLV-associated diseases include a variety of neoplastic disorders, anemia, leucopenia, thrombocytopenia, neurological disorders, reproductive failure in female cats, and numerous secondary infections caused by FeLV-induced immunosuppression [12,34]. Although the period of disease progression is highly variable, 83% of FeLV-infected cats die within 3 years [29].
Given these findings, the accurate diagnosis of FeLV infection is very important to break the cycle of horizontal and vertical transmission in feline populations. Several diagnostic tools have been introduced in veterinary clinics: the passive hemagglutination test [37], complement fixation test [36], immunofluorescent assay [13], enzyme linked-immunosorbent assay (ELISA) [25,26,27,28], saliva test [10], and rapid diagnostic test (RDT) [7]. The RDT, also known as the lateral flow rapid test, has several advantages such as quick turnaround, cost-effectiveness, and usability in locations far removed from laboratories. Consequently, the RDT has been widely used in clinics and elsewhere [30,39]. In this report, we describe the preparation of monoclonal antibodies specific for the p27 of FeLV. We also provide details about the development of an RDT system using these antibodies and clinical characteristics of the assay.
Feline leukemia virus (VR-719), feline immunodeficiency virus (FIV, VR-1312), feline panleukopenia virus (FPV, VR-2017), feline coronavirus (FCoV, VR-2004), feline calicivirus (FCaV, VR-782), canine adenovirus (CAV, VR-293), canine coronavirus (CCV, VR-809), and canine distemper virus (CDV, VR-1587) were purchased from the American Type Culture Collection (USA). Canine parvovirus Type 2a VI (CPV, KVCC-VR0900161) was kindly provided by the National Veterinary Research and Quarantine Service (Korea). Additionally, 282 sera samples from 155 household felines and 127 stray cats were provided by the National Veterinary Research and Quarantine Service as well as four different animal hospitals in Chungbuk province (Korea): Jeonju Animal Hospital, Woori Animal Hospital, Soo Animal Hospital, and Juju Animal Hospital. The samples were collected from December 2009 to March 2012.
Vero cells (kindly provided by Professor Chan Hee Lee, Chungbuk National University, Korea) were used for FeLV culture. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen) at 37℃ in 5% CO2. Before infection with the virus, the cells were washed with phosphate-buffered saline (PBS) and inoculated with the viruses for 6 h in DMEM containing 5% FBS at 37℃ in 5% CO2. After 4 days, the culture media was changed and the infection progressed until 80~90% of the cells were floating or lightly attached to the T-75 flask (typically 10 days post-infection) (Thermo Scientific, USA). The viruses were harvested and purified by density gradient ultracentrifugation (3% sucrose) as previously described [32].
To clone the genes encoding p27 in FeLV, two primers were designed and synthesized (Cosmogenetech, Korea). The forward primer was 5'-gaattccccttgagggagggcccca acaac-3' (the BamHI site is underlined) and the reverse primer was 5'-ctcgagcagaactttagtcatctccttgtg-3' (the XhoI site is underlined). Reverse transcription was carried out using the viral genome under the following conditions: 90 min at 37℃, 5 min at 94℃, and holding at 4℃. Polymerase chain reaction (PCR) amplification of the p27 gene was performed under the following conditions: 35 cycles consisting of 1 min 30 sec at 94℃, 1 min 30 sec at 59℃, and 1 min 30 sec at 72℃. Each reaction mixture contained 100 ng of template, 10 pmole of two primers, 100 mM KCl, 200 mM Tris (pH 8.8), 100 mM (NH4)2SO4, 20 mM MgSO4. The 756 base pair (bp) PCR product was cloned into a pGEM-T vector (Promega, USA) for sequencing by Cosmogenetech and the data were analyzed by ClustalW ver. 2.0 [23].
The gene insert in the pGEM-T vector was subcloned into a pET28a expression vector (Merk Millipore, USA) and used to transformed CaCl2-competent E.coli BL21(DE3) cells (Promega). The transformants were grown at 37℃ in Luria-Bertani medium (Difco; Becton Dickinson and Company, USA) to an optical density at 600 nm (OD600) of 0.7~0.8 by DU UV/Vis Spectrophotometer (Beckman Coulter, USA).
Expression of the recombinant protein was induced with 1 mM isopropyl-β-D-thiogalatoside (Thermo Scientific) for 5 h at 30℃ and the cells were then harvested by centrifugation at 5,000 × g 20 min at 4℃. The cell pellets were resuspended in a standard buffer (50 mM Tris-HCl (pH 7.9) and 500 mM NaCl) containing 1 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich, USA). The suspension was then lysed by sonication (Vibra-Cell; Sonics & Materials, USA) under ice-cold conditions (15 min with 5 sec on/5 sec off cycles). The homogenate was centrifuged at 20,000 × g for 20 min at 4℃ and the supernatant was then subjected to metal affinity chromatography using Ni-NTA agarose (Qiagen, Germany). The cell lysate was added into a column that had been equilibrated with standard buffer. Unbound proteins were removed by washing with a sufficient volume of washing buffer (standard buffer containing 20 mM imidazole (Sigma-Aldrich). Next, 6xHis-tagged recombinant protein was recovered from the column with elution buffer (standard buffer containing 500 mM imidazole). The isolated protein was dialyzed at 4℃ with 10 mM sodium carbonate (pH 9.0) (Sigma-Aldrich) and identified by SDS-PAGE analysis [22]. The protein concentration was measured by the method developed by Bradford [21].
Hybridoma technology was used to manufacture anti-FeLV p27 antibodies as previously described [20]. Hybridomas producing monoclonal antibodies (mAbs) against the core p27 protein were generated as follows. Spleen cells from BALB/c mice (7-week-old females; DBL, Korea) immunized with purified rec. p27 were fused to sp2/0 myeloma cells. Rec. p27 was mixed with an equivalent volume of complete Freund's adjuvant (Sigma-Aldrich) for the first immunization and with same volume of incomplete Freund's adjuvant (Sigma-Aldrich) for the second and third rounds of immunization. Immunizations were carried out at 2-week intervals by injecting the mice with 0.5 mL (50 µg) of rec. p27. The anti-p27 antibody titer was measured by an ELISA using plates coated with 1 µg/mL of purified FeLV. When the optical density at 450 nm (OD450) was greater than 0.2, the next boosting step was conducted. The boosting immunization was performed by injecting the mice with 0.2 mL (50 µg) of rec. p27 via the tail vein. After 3 days, cell fusion with sp2/0 myeloma cells was performed according to the method of Köhler and Milstein. Based on the screening steps using plates coated with rec. p27 and the purified FeLV virus, hybridomas producing the antibodies of interest were selected and subcloned from a single cell using the limited dilution method. The hybridomas were used to produce ascitic fluid in the BALB/c mice, and the immunoglobulin G (IgG) was purified with a protein G-coupled Sepharose column (GE Healthcare, USA). Isotyping of the cloned mAbs was carried out using goat anti-mouse IgG (Sigma-Aldrich). Among the antibodies produced by the hybridoma, 8F8B5 was selected as the capture antibody and 8G7D1 was selected for conjugation. These antibodies were specific for FeLV p27 and had the highest affinity constants (Table 1).
Colloidal gold particles (40 nm mean diameter) were prepared according to a previously described procedure [8]. Briefly, 100 mL of a 0.01% HAuCl4 solution (Sigma-Aldrich) in a 250 mL conical beaker was boiled thoroughly, and 1.8 mL of 1% trisodium citrate solution (Sigma-Aldrich) was then added with constant stirring. After the color of the solution changed to wine-red after about 45 sec, the solution was boiled for another 5 min. The heat source was removed and the colloidal gold solution was stirred for another 10 min. The solution was stored in a dark bottle at 4℃ and used to prepare the colloidal gold-mAb conjugate as soon as possible. Anti-p27 mAb 8G7D1 was conjugated with the 40-nm colloidal gold particles as previously described [5,6,37]. The mAb 8G7D1 was subsequently dialyzed with 1 mM Borax (pH 9.0) (Sigma-Aldrich) for 5 h at 4℃. The pH of the gold solution was adjusted to 9.0 with 0.2 M K2CO3 and mixed with 1 mg/mL of mAb. The mixture was then incubated for 30 min at room temperature. The gold conjugate was blocked with 1% bovine serum albumin (BSA; Sigma-Aldrich) for 30 min under at room temperature. The conjugate was then washed three times with PBS containing 1% BSA. The OD of the mAb-gold conjugate was measured at 540 nm by spectrophotometer.
The RDT strips were prepared as follows: 1.0 mg/mL of anti-p27 mAb 8F8B5 was dispensed and immobilized to the test line zone of a nitrocellulose membrane (MDI, India). Next, 1.0 mg/mL of goat anti-mouse IgG (Arista Biologicals, USA) was added to the control line zone. The mAb 8G7D1-gold conjugate was dispensed and dried on a glass fiber (Merck Millipore) for use as the conjugate pad (4.0 OD/pad). The sample pad was prepared by treating cellulose paper (Merck Millipore) with 0.1 M sodium carbonate (pH 9.3) containing 1% Triton X-100 and 0.5% casein (Sigma-Aldrich). The absorbance pad consisted of cellulose paper alone. The test strip was assembled and all pads were partially overlapped to enable migration of the sample solution along the test strip.
The RDT was started by loading 10 µL of blood specimen into the hole of the cassette device, and then loading three drops (approximately 100 µL) of 0.1 M carbonate buffer (pH 9.0) containing 0.1% casein and 1% Tween-20 onto the sample pad. Approximately 20 min after application of the buffer, the results were interpreted. The control line was expected to appear in all tests. If a red band appeared at the test line, the specimen contained FeLV antigen.
To confirm FeLV infection, viral DNA was isolated from the cat blood samples using Genomic DNA preparation kit (Qiagen) and PCR was performed with a commercially available FeLV Detection Kit (GeNet Bio, Korea). The kit is designed to amplify conserved fragment of the FeLV U3 long terminal repeat [16]. Total genomic DNA was directly extracted from the blood specimens using a Genomic DNA preparation kit (Qiagen). Target DNA was amplified by 35 cycles of PCR with three steps at 94℃ for 1 min for denaturation, 50℃ for 1 min for annealing, and 72℃ for 2 min for elongation. Finally, a further elongation step (72℃ for 5 min) was performed. The 166 bp fragment of amplified gene product was analyzed with 2% agarose gel electrophoresis.
A total of 282 feline serum specimens (from 155 household felines and 127 stray cats) were used to evaluate the RDT developed in this study. All samples were tested by PCR analysis to confirm FeLV infection. Clinical characteristics of the RDT kit including sensitivity, specificity, and positive or negative predictive values (PPV or NPV) were calculated using standard formulae [4,18]. Sensitivity was expressed as the percentage (%) of positive test results obtained for all samples containing FeLV according to the PCR analysis. Specificity was calculated as the % of negative test results obtained from samples with negative PCR results. PPV was the proportion of true positive results among all positive samples and NPV was the proportion of true negative results among all negative samples.
As shown in Fig. 1A, we amplified a 756 bp fragment of the p27 gene from FeLV culture media. The gene was subcloned into the pET28a expression vector and successfully expressed as a soluble protein. Homogenous rec. p27 was purified as shown in Fig. 1B. A commercially available ELISA (SNAP FeLV Ag/FIV Ab Combo; Idexx Laboratories, USA) demonstrated that the purified rec. p27 had very strong antigenic activity (Fig. 2).
We screened several clones that secreted antibodies which reacted to rec. p27 antigen with different affinities. We were able to obtain 10 clones that produced antibodies with high affinities for rec. p27 (Table 1). As shown in Fig. 3, antibodies from four hybridomas (1C5E1, 1H12A5, 6C2, and 9H3A6) had good reactivity against rec. p27 but did not recognize the purified FeLV. The mAbs 2B2B2 and 2B3D1 bound strongly to rec. p27 but weakly with the purified virus. The remaining four hybridomas (3B5C2, 1H8G10, 8F8B5, and 8G7D1) appeared to react to both rec. p27 and the purified virus. Of these, clones 8F8B5 and 8G7D1 had higher affinities (Ka; 0.35 × 109 and 0.86 × 109, respectively; Table 1). No non-specific reactions against FeLV-negative blood or FIV, CAV, CCV, CDV, and CPV were observed. Antibody produced by hybridoma 1H8G10 reacted very strongly against rec. p27. However, this was problematic since the hybridoma produced ascitic fluid because it formed a solid tumor in the mouse. We also excluded hybridoma 3B5C2 due to the lower affinity of the antibody produced compared to that generated by 8F8B5 and 8G7D1. Ultimately, antibodies produced by hybridomas 8F8B5 and 8G7D1 were selected for capturing in the membrane and as the gold conjugate for the RDT kit. In our experiments, mAb 8F8B5 was effective as a capturer and mAb 8G7D1 performed well as a conjugator.
To identify the detection limits of the RDT kit, the analytic sensitivity was measured with different concentrations of rec. p27 protein and FeLV culture media. As shown in Fig. 4, the detection threshold of the RDT kit for rec. p27 was 2 ng/mL and 12.5 × 104 IU/mL for the virus. This is an excellent level of sensitivity and higher or comparable to the previously reported ones. The cross-reaction test demonstrated that the RDT kit did not produce any false-positives to other feline infectious viruses including FIV, FPV, FCoV, and FCaV (data not shown).
A total of 282 feline specimens were simultaneously tested using the RDT kit. Presence of FeLV infections in the samples was confirmed by conventional PCR. As shown in Table 2, the RDT kit had a sensitivity of 95.2% (20/21) and a specificity of 98.5% (257/261; p < 0.001) assuming that the PCR results were correct. These levels of accuracy, expressed as percentages, were calculated as the number of positive or negative RDT results divided by the number of positive or negative PCR results. The positive and negative predictive values associated with the RDT kit were 83.3% (20/24) and 99.6% (257/258), respectively.
As shown in Fig. 1, the molecular size of purified rec. p27 was approximately 31 kDa, which resulted from the 27 kDa insert plus 4 kDa tag protein. Antigenicity of the rec. p27 was tested using a commercially available ELISA kit. According to the hybridoma screening results, almost all clones produced antibodies reactive to rec. p27. A few clones including 1C5E1, 1H12A5, 6C2, and 9H3A6 generated antibodies with reactivity to the purified virus with different binding affinities, indicating that the rec. p27 might have a structure very similar to that of native p27. A commercial ELISA kit that detected FeLV antigen demonstrated that the rec. p27 was very strongly positive (data not shown). Clones that were non-reactive to the purified virus perhaps poesses their epitopes to be tag protein or specific conformational structure of viral capsid. In the pairing test, the best result was obtained when 8F8B5 was the gold conjugate and 8G7D1 was the capturer. Otherwise, the sensitivity of the RDT was suboptimal or very low. Certain parameters such as steric hindrance may have affected antigen-antibody binding.
According to a previous report [28], the mean concentration of FeLV p27 in infected feline serum from a cat suffering from persistent viremia was 4,800 ng/mL (600~15,000 ng/mL) and 3,600 ng/mL (300~28,000 ng/mL) in serum from a feline with transient viremia. Therefore, the RDT using 8F8B5 and 8G7D1 which had a detection limit of 2 ng/mL for rec. p27 and 12.5 × 104 IU/mL for the virus would be very useful for practical diagnosis. According to other previous reports [14,15], the diagnostic sensitivities and specificities of current commercialized diagnostic kits including enzyme immunoassays (EIAs) range from 92.0~94.7% and 97.5~99.8%, respectively. The RDT system is typically believed to be less sensitive than an EIA because RDT does not include a washing step and various blocking reagents are used to prevent non-specific reactions. The RDT kit developed in the present study was found to possess excellent diagnostic properties (a sensitivity of 95.2% and specificity of 98.5%). Compared to the BioNote Rapid FeLV/FIV Combo Kit (cat. no: RC12-04, lot no: 1204172; Alere BioNote, Korea), the RDT we developed showed a lower level of analytical sensitivity (data not shown). Using 12 FeLV-positive specimens, both RDTs identified all samples as positive but the band signal of the RDT we produced was stronger than that of the commercial RDT (data not shown). We speculate that the high accuracy of our RDT was due to the properties of the monoclonal antibodies (8F8B5 and 8G7D1) with high affinity constants that we generated.
The RDT can serve as a point-of-care testing (POCT) tool because of its rapid assay time, ease of use, and no need for specialized machines or expert technicians. A POCT for FeLV infection is important because it would facilitate field-testing of cats, especially stray animals, by veterinarians. In our study, none of the 155 household cats were FeLV-positive while 21 out of 127 stray cats produced positive results. To prevent the spread of FeLV infection among cat populations, stray animals should be rapidly diagnosed and treated. The RDT developed in our study could be used as a promising POCT tool for diagnosing FeLV infection in conjunction with other conventional antigen tests such as ELISAs [25,26,27,28] and nucleic acid testing [1,2,3].
Figures and Tables
Fig. 1
(A) Cloning of the FeLV p27 gene. (B) Expression and purification of soluble p27 protein. M, (A) DNA size markers; (B) protein size markers; Lane 1, insoluble fraction from the cell lysate; Lane 2, soluble fraction from the cell lysate; Lane 3, purified recombinant p27 (rec. p27). S: sample.
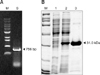
Fig. 4
Analytical sensitivity of the rapid diagnostic test (RDT) using rec. p27 protein (A) and FeLV particles (B). IU/mL: infectious units per mL, +W: weakly positive.

Table 1
Properties of hybridomas produced in this study

*Affinity constant = 1/dissociation constant (Kd) where Kd was determined by a Klotz plot according to the method described by Friguet et al. [9]. NM: not measured.
Acknowledgments
This work was supported by Daejeon Health Sciences College (Korea) in 2012 (Grant No. 2012013).
References
1. Arjona A, Barquero N, Doménech A, Tejerizo G, Collado VM, Toural C, Martín D, Gomez-Lucia E. Evaluation of a novel nested PCR for the routine diagnosis of feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV). J Feline Med Surg. 2007; 9:14–22.

2. Cattori V, Hofmann-Lehmann R. Absolute quantitation of feline leukemia virus proviral DNA and viral RNA loads by TaqMan real-time PCR and RT-PCR. Methods Mol Biol. 2008; 429:73–87.

3. Cattori V, Tandon R, Pepin A, Lutz H, Hofmann-Lehmann R. Rapid detection of feline leukemia virus provirus integration into feline genomic DNA. Mol Cell Probes. 2006; 20:172–181.

5. Cramer EM, Beesley JE, Pulford KAF, Breton-Gorius J, Mason DY. Colocalization of elastase and myeloperoxidase in human blood and bone marrow neutrophils using a monoclonal antibody and immunogold. Am J Pathol. 1989; 134:1275–1284.
6. De Waele M, Renmans W, Segers E, De Valck V, Jochmans K, Van Camp B. An immunogold-silver staining method for detection of cell surface antigens in cell smears. J Histochem Cytochem. 1989; 37:1855–1862.

7. Eto N, Yazaki-Takayama N, Takayama Y, Yoshino-Nakamura T, Kobayashi Y. Immuno-chromatographic assay for diagnosis of feline leukemia virus infection. Cytotechnology. 2003; 43:65–72.

8. Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature. 1973; 241:20–22.

9. Friguet B, Chaffotte AF, Djavadi-Ohaniance L, Goldberg ME. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J Immunol Methods. 1985; 77:305–319.

10. Gomes-Keller MA, Gönczi E, Tandon R, Riondato F, Hofmann-Lehmann R, Meli ML, Lutz H. Detection of feline leukemia virus RNA in saliva from naturally infected cats and correlation of PCR results with those of current diagnostic methods. J Clin Microbiol. 2006; 44:916–922.

11. Hardy WD Jr, Hess PW, Essex M, Cotter S, McClelland AJ, MacEwen G. Horizontal transmission of feline leukemia virus in cats. Bibl Haematol. 1975; 40:67–74.

12. Hardy WD Jr, Hess PW, MacEwen EG, McClelland AJ, Zuckerman EE, Essex M, Cotter SM, Jarrett O. Biology of feline leukemia virus in the natural environment. Cancer Res. 1976; 36:582–588.
13. Hardy WD Jr, Hirshaut Y, Hess P. Detection of the feline leukemia virus and other mammalian oncornaviruses by immunofluorescence. Bibl Haematol. 1973; 39:778–799.
14. Hartmann K, Griessmayr P, Schulz B, Greene CE, Vidyashankar AN, Jarrett O, Egberink HF. Quality of different in-clinic test systems for feline immunodeficiency virus and feline leukaemia virus infection. J Feline Med Surg. 2007; 9:439–445.

15. Hartmann K, Werner RM, Egberink H, Jarrett O. Comparison of six in-house tests for the rapid diagnosis of feline immunodeficiency and feline leukaemia virus infections. Vet Rec. 2001; 149:317–320.

16. Hofmann-Lehmann R, Huder JB, Gruber S, Boretti F, Sigrist B, Lutz H. Feline leukemia provirus load during the course of experimental infection and in naturally infected cats. J Gen Virol. 2001; 82:1589–1596.

17. Hoover EA, Mullins JI. Feline leukemia virus infection and diseases. J Am Vet Med Assoc. 1991; 199:1287–1297.
18. Jacobson RH. Validation of serological assays for diagnosis of infectious diseases. Rev Sci Tech. 1998; 17:469–526.

19. Jarrett WFH, Crawford EM, Martin WB, Davie F. A virus-like particle associated with leukaemia (lymphosarcoma). Nature. 1964; 202:567–568.

20. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975; 256:495–497.

22. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970; 227:680–685.

23. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007; 23:2947–2948.

24. Leutenegger CM, Hofmann-Lehmann R, Riols C, Liberek M, Worel G, Lups P, Fehr D, Hartmann M, Weilenmann P, Lutz H. Viral infections in free-living populations of the European wildcat. J Wildl Dis. 1999; 35:678–686.

25. Lutz H, Pedersen NC, Durbin R, Theilen GH. Monoclonal antibodies to three epitopic regions of feline leukemia virus p27 and their use in enzyme-linked immunosorbent assay of p27. J Immunol Methods. 1983; 56:209–220.

26. Lutz H, Jarret O. Detection of feline leukemia virus infection in saliva. J Clin Microbiol. 1987; 25:827–831.

27. Lutz H, Pedersen NC, Higgins J, Harris CW, Theilen GH. In : Hardy WD, Essex M, McClelland AJ, editors. Quantitation of p27 in the serum of cats during natural infection with feline leukemia virus. Feline leukemia virus: proceedings of the Third International Feline Leukemia Virus Meeting; May 5-9, 1980; St. Thomas, United States Virgin Islands. Amsterdam: Elsevier;1980. p. 497–505.
28. Lutz H, Pedersen NC, Theilen GH. Course of feline leukemia virus infection and its detection by enzyme-linked immunosorbent assay and monoclonal antibodies. Am J Vet Res. 1983; 44:2054–2059.
29. McClelland AJ, Hardy WD Jr, Zuckerman EE. In : Hardy WD, Essex M, McClelland AJ, editors. Prognosis of healthy feline leukemia virus infected cats. Feline leukemia virus: proceedings of the Third International Feline Leukemia Virus Meeting; May 5-9, 1980; St. Thomas, United States Virgin Islands. Amsterdam: Elsevier;1980. p. 121–126.
30. Noyola DE, Demmler GJ. Effect of rapid diagnosis on management of influenza A infections. Pediatr Infect Dis J. 2000; 19:303–307.

31. Pedersen NC, Theilen G, Keane MA, Fairbanks L, Mason T, Orser B, Che CH, Allison C. Studies of naturally transmitted feline leukemia virus infection: changes occurring during the initial stage of infection. Am J Vet Res. 1977; 38:1523–1531.
32. Reimer CB, Baker RS, Newlin TE, Havens ML. Influenza virus purification with the zonal ultracentrifuge. Science. 1966; 152:1379–1381.

33. Reinacher M. Feline leukemia virus-associated enteritis - a condition with features of feline panleukopenia. Vet Pathol. 1987; 24:1–4.

34. Reinacher M, Theilen G. Frequency and significance of feline leukemia virus infection in necropsied cats. Am J Vet Res. 1987; 48:939–945.
35. Rogerson P, Jarrett W, Mackey L. Epidemiological studies on feline leukaemia virus infection. I. A serological survey in urban cats. Int J Cancer. 1975; 15:781–785.

36. Sarma PS, Gilden RV, Huebner RJ. Complement-fixation test for feline leukemia and sarcoma viruses (the COCAL test). Virology. 1971; 44:137–145.

37. Sibal LR, Fink MA, Plata EJ, Kohler BE, Noronha F, Lee KM. Methods for the detection of viral antigen and antibody to a feline leukemia virus (a preliminary report). J Natl Cancer Inst. 1970; 45:607–612.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download