Introduction
Embryonic stem cells (ESCs) have the unique potential to differentiate into any cell type. A number of studies have demonstrated their pluripotency in vitro including the ability to differentiate into hepatocytes [24], cardiomyocytes [34], and neurons [18]. These cells are therefore potentially important for cell therapy in the field of regenerative medicine, and are a useful model for assessing the toxicity of new drugs and pharmacological chemicals.
Recently, the embryonic stem cell test (EST) has been used as an in vitro alternative to animal testing and a new predictive model for risk assessment. An EST using ESCs and 3T3 fibroblasts has been validated for use in assessing the embryotoxic potential of chemicals. An inhibition assay involving ESC-derived cardiomyocyte differentiation has also been developed [12,13]. Finally, an EST using functional neuron-like cells derived from ESCs has been established as a neurotoxicity test [30,31]. The European Centre for the Validation of Alternative Methods (ECVAM) has encouraged further optimization of EST-based methods as novel toxicological prediction models.
In vitro neural development models have recently been established for mouse and human cells. ESCs can efficiently differentiate into neural progenitor cells and neural lineage cells. Neural progenitor cells derived from the neuroectoderm can also differentiate into neuronal- and glial-restricted precursor cells [22]. Various approaches have been used to promote the neural differentiation of ESCs [3,17,38]. Basically, all methods are designed to recreate the multistep differentiation process that occurs during in vivo neurogenesis.
During embryogenesis, a large number of tissue-specific genes systemically interact within the normal microenvironment to direct specific cell fates. During ESC-derived neurulation, the expression of neural-specific genes can be used to confirm the developmental status of the cells. For example, Nestin is exclusively expressed in neural progenitor cells [39] while the expression of gamma-aminobutyric acid receptor (GABAA-R), glial fibrillary acidic protein (GFAP), neuron-specific class III beta-tubulin (Tuj1), and microtubule associated protein (MAP2) is observed in more committed neural cells such as neurons, astrocytes, and oligodendrocytes [10,20,21]. In studies of cell-based toxicity, quantitative changes of molecules such as RNA and proteins resulting from chemical exposure can serve as a useful indication of cytotoxicity in neuronal cells [30,31].
Methylmercury (MM) is a well-known neurotoxin that causes neural defects [1]. Therefore, MM could be used as reference compound to evaluate neurotoxic effects according to the quantification of neural proteins. Arsanilic acid (AA) and danofloxacin (DF) were developed for agricultural and veterinary medicine, and are widely used in the livestock industry. The neurotoxic effects of these two compounds on neural development in vitro have been evaluated. Arsenic is a widespread contaminant in the environment. In particular, inorganic arsenic exerts severe teratogenic and embryotoxic effects [11,36]. Despite these data, there is a lack of available evidence demonstrating organic AA-induced neurotoxicity. Likewise, there is no evidence that DF, one of the fluoroquinolones (FQs), causes neural developmental defects even though other FQs have neurotoxic effects [8].
The purpose of the present study was to investigate the chemical-induced neurotoxic effects of MM, AA, and DF during neural differentiation by measuring the expression of neural-specific proteins. We induced the differentiation of mouse ESCs into neural cells via adherent culture conditions. The differentiating and differentiated neural cells were subsequently exposed to each of the three chemicals. Changes of expression for several neural-specific proteins resulting from exposure to the chemicals were measured using fluorescence-based assays at several time points following treatment. Additionally, neurotoxic effects of the chemicals were evaluated by measuring the activity of acetylcholinesterase (AChE).
Materials and Methods
Chemicals
MM (MeHg), AA (p-aminophenylarosonic acid), and DF were purchased from the Sigma-Aldrich Chemical Company (USA). All three chemicals were dissolved in Dimethyl sulfoxide (DMSO), and final concentrations were adjusted with neural differentiation medium. A DMSO concentration of 0.5% was used as a control.
Mouse ESC culturing and neural differentiation
Mouse ESCs established in 2006 in our laboratory and referred to as NVRQS-11F [16] were maintained using a conventional ESC culture protocol. Briefly, the ESCs were cultured on mitomycin C-treated mouse embryonic fibroblast feeder cells in 0.1% gelatin-coated dishes with Dulbecco's modified Eagle's medium (DMEM; Millipore, USA) supplemented with 15% fetal bovine serum (FBS; Invitrogen, USA), 2 mM L-glutamine (Millipore), 1% non-essential amino acids (Invitrogen), 1% 2-mercaptoethanol (Millipore), 1% nucleoside (Millipore), 1% penicillin-streptomycin (Millipore), and 10 ng/mL mouse leukemia inhibitory factor (Millipore). To induce differentiation of the ESCs into neural cells, 5 × 103 ESCs were seeded on 0.1% gelatin-coated 12-well plates (TPP Techno Plastic Products, Switzerland). The ESCs were cultured in a mixture (1 : 1) of neural basal medium (Invitrogen) supplemented with 1% N2 (Invitrogen) or DMEM/F12 (Invitrogen) supplemented with 2% B27 (Invitrogen) during differentiation for 14 days. The media were changed every other day.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay
To determine the non-cytotoxic concentrations of the test chemicals, the ESCs were exposed to 0 to 80 µM of MM, 0 to 25 mM of AA, and 0 to 1 mM for DF of 16 h at 37℃. Cell viability was measured with an MTT assay (ATCC, USA). After the inhibitory concentration 15 (IC15) for each chemical was determined by the assay, serial dilutions (MM; 0 to 1,000 nM, AA; 0 to 4 mM, DF; 0 to 80 µM) of the three chemicals using the IC15 as the highest dose were made to evaluate the neurotoxic effects of these agents at non-cytotoxic doses on the differentiation of ESCs into neural cells.
Immunofluorescence assay
To detect the expression of marker proteins indicative of ESC differentiation into neural cells, immunocytochemical staining for POU5F1 (Oct4), GABAA-R, GFAP, Nestin (intermediate filament protein), Tuj1, and MAP2 was performed on cells grown in culture dishes. The cell culture medium was removed by aspiration, and the cells were washed twice with 1× phosphate buffered saline (PBS; Invitrogen), pH 7.4. The cells were fixed with 4% paraformaldehyde (Sigma-Aldrich) for 5 min at room temperature and then rinsed twice with 1× PBS. After the cells were permeabilized with 1× PBS containing 0.1% Triton X-100 (PT) for 10 min at room temperature, 4% normal goat serum (Abcam, USA) in PT was added as a blocking solution. After blocking for 30 min at 4℃, anti-Oct4 (Millipore), anti-GABAA-Rα1 (Millipore), anti-GFAP (Millipore), anti-Nestin (Santa Cruz Biotechnology, USA), anti-Tuj1 (Millipore), or anti-MAP2 (Millipore) monoclonal antibodies were applied at a 1 : 100 dilution for 1 h at room temperature. The cells were then incubated with fluorescein isothiocyanate-conjugated anti-rabbit or anti-mouse IgG/IgM (1 : 100; Millipore) for 30 min at room temperature. Immunofluorescence was detected using a fluorescence microscope (Axiovert; Carl Zeiss, Germany), and quantitation of the marker proteins was performed with ArrayScan VTI HCS (Thermo Scientific, USA) after counterstaining with Hoechst 33258 (Invitrogen). All fluorescent signals were corrected by dividing the fluorescence intensity by the number of cells.
AChE assay
To evaluate the effects of MM, AA, and DF on the production of acetylcholine, the ESCs were exposed to non-cytotoxic doses of each chemical under two conditions. First, the cells were exposed to the chemicals during the 8 days of differentiation (differentiating stage). The cells were also exposed to the chemicals for 6 days starting after 8 days of differentiation (differentiated stage). AChE activity was measured following the manufacturer's instructions (Molecular Probes, USA). Briefly, culture media from treated and untreated cells were collected from 12-well plates and diluted with reaction buffer. Next, 100 µL of the diluted samples and controls was transferred to a microplate and 100 µL of Amplex Red reagent containing horseradish peroxidase/choline oxidase/acetylcholine was added. After incubation at room temperature for 30 min, fluorescence (excitation at 530~560 nm and emission at 590 nm) was measured by FlexStation 3 (Molecular Devices, USA). Enzyme activity was normalized by subtracting the values for the negative control and the results are presented as percentage of the control values for each concentration.
Statistical analysis
All data are expressed as the mean ± standard error (SE) of three independent replicate experiments. Statistical significance was identified using STATISTICA 5.5 (StatSoft, USA) with a one-way analysis of variance (ANOVA) and post hoc comparisons between the control group and each treatment group using Duncan's multiple comparison tests. A p value < 0.05 was considered statistically significant.
Results
Quantitative change of neural-related protein expression during differentiation
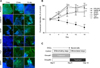
Undifferentiated ESCs appeared tight, round, and multi-layered. The cells also morphologically changed and adopted neurite-like shapes during the differentiation step. To confirm neural differentiation, the expression of POU5F1 (a marker of undifferentiated ESCs) along with GABAA-R, GFAP, Nestin, Tuj1, and MAP2 (as neural lineage markers) was confirmed during the 14 days of differentiation (Fig. 1A). Fluorescent intensities corresponding to these factors were measured on Day 0, 4, 8, 12, and 14 (Fig. 1B). As shown in Fig. 1B, POU5F1 levels gradually decreased during differentiation. Meanwhile, the expression of GABAA-R and Nestin was significantly increased (p < 0.01) by Day 8. The expression of GFAP, Tuj1, and MAP2 was also significantly increased (p < 0.01) by Day 4 and continued to increase until Day 8. The levels of these proteins were then maintained until Day 14.
In this in vitro neural differentiation system, ESCs might be completely differentiated into neural cells by Day 8 under our neural induction conditions. Based on the expression of five neural-specific proteins, we chose Day 8 as the critical point of differentiation. Therefore, we established two treatment groups for subsequent experiments to evaluate the neurotoxic effects of the three chemicals on the different stages of neural development. Each chemical was administered during the differentiating stage (Days 1 through 8) as well as the differentiated stage (Days 9 through 14) (Fig. 1C).
Effects of the test chemicals at non-cytotoxic concentrations
To identify the appropriate test dose, we evaluated the cytotoxicity of the three chemicals using serial dilutions of the compounds (160~80,000 nM for MM, 0.049~25 mM for AA, and 1.95~1,000 µM for DF). The ESCs were exposed to the reagents for 16 h, and cell viability was estimated using an MTT assay (Fig. 2). IC15 values were 1,000 nM for MM, 4 mM for AA, and 80 µM for DF (Figs. 2A-C). The cells were exposed to the serially diluted, non-cytotoxic concentrations of each chemical during the differentiating and differentiated stages using the respective IC15 values of each chemical as the highest dose.
Quantitative analysis of marker protein expression
MM treatment
To evaluate the effects of MM during the differentiating and the differentiated stage, the expression levels of marker proteins were compared to those found in the control (Fig. 3). POU5F1 expression was significantly increased (p < 0.05) by MM at concentrations greater than 250 nM during the differentiating stage and greater than 125 nM during the differentiated stage (Fig. 3A). GABAA-R expression during the differentiated stage and Nestin levels in the differentiating stage were markedly decreased (p < 0.05) by lower doses compared to their counterparts (differentiating stage vs. differentiated stage: 500 nM vs. 15.13 nM for GABAA-R and 15.13 nM vs. 250 nM for Nestin; Figs. 3B and D). The expression of GFAP, Tuj1, and MAP2 was significant different in cells treated with high doses of MM compared to the control (differentiating stage vs. differentiated stage: 250 nM vs. 500 nM for GFAP, 62.5 nM vs. 125 nM for Tuj1, 500 nM vs. 250 nM for MAP2, p < 0.05; Figs. 3C, E, and F).
AA treatment
As shown in Fig. 4, the expression of POU5F1 was significantly increased (p < 0.05) by concentrations of AA greater than 0.125 mM during the differentiating stage and 0.5 mM during the differentiated stage (Fig. 4A). This chemical also restricted ESC differentiation similar to MM. Cells in the differentiating stage were relatively resistant to the effects of AA on GABAA-R and GFAP while those in the differentiated stage required lower levels for similar effects (differentiating stage vs. differentiated stage: 0.5 mM vs. 0.062 mM for GABAA-R and 1 mM vs. 0.062 mM for GFAP, p < 0.05; Figs. 4B and C). However, the differentiating cells exhibited changes in Nestin and Tuj1 expression at lower AA concentrations than the differentiated cells (differentiating stage vs. differentiated stage: 0.125 mM vs. 0.25 mM for Nestin and 0.125 mM vs. 0.5 mM for Tuj1, p < 0.05; Figs. 4D and E). MAP2 expression was significantly decreased (p < 0.05) during both stages with doses of AA greater than 0.5 mM (Fig. 4F).
DF treatment
Overall, DF exerted less toxic effects during both stages compared to the other chemicals (Fig. 5). At relatively high concentrations, DF increased POU5F1 expression during the differentiated stage more than the differentiating stage (10 µM vs. 40 µM; Fig. 5A). GABAA-R seemed to be affected by high doses during the differentiating stage while the expression levels during the differentiated stage were significantly decreased (p < 0.05) by DF at concentrations greater than 10 µM (Fig. 5B). GFAP and Tuj1 expression during the differentiated stage was significantly decreased by all concentrations of DF, but this effect was observed only at concentrations greater than 5 and 10 µM during the differentiating stage (Figs. 5C and E). The production of Nestin was significantly decreased (p < 0.05) by concentrations of DF greater than 10 µM during both stages (Fig. 5D). MAP2 expression during the differentiated stage was more sensitive to DF than during the differentiating stage (5 µM vs. 20 µM, p < 0.05; Fig. 5F).
AChE assay results
AChE activity was monitored in the neural cells. The levels of enzyme activity were estimated for each control and compared to that of the treatment groups during the differentiating and the differentiated stage to evaluate the effects of the three chemicals on acetylcholine production (Fig. 6). AChE levels were significantly different between the controls samples during the differentiating and differentiated stages (Fig. 6A). This result could indicate that AChE levels were greatly increased in the fully differentiated cells (after Day 8). Relative to the stage-specific control, the activities of AChE were generally decreased in a concentration-dependent manner by almost all chemicals. Specifically, the activity of AChE was inhibited by exposure to all concentrations of MM during the differentiating stage, MM concentrations greater than 125 nM during the differentiated stage (Figs. 6B and E), levels of AA greater than 0.5 mM during the differentiating stage, and AA concentrations more than 4 mM during the differentiated stage (Figs. 6C and F). However, DF treatment resulted in significant differences (p < 0.01) at concentrations greater than 40 µM during the differentiated stage while there were no significant differences induced by this chemical during the differentiating stage (Figs. 6D and G).
Discussion
In vitro neurotoxic testing can be a valuable alternative method for risk assessment of toxicants and preclinical drug testing. After exposure to chemicals and drugs, this in vitro alternative assay can detect cellular alterations caused by toxicants that affect genetic (e.g., changes to DNA and RNA expression) or cellular (e.g., the induction of apoptosis, alteration of cell growth, and secretion of cell-specific proteins) functions. Therefore, in vitro testing might be a powerful method for predicting chemical toxicity during neural development. In the present study, we hypothesize that the toxic effects of three different chemicals might differ between cells under ongoing differentiation and ones completely committed. To test our hypothesis, ESCs underwent in vitro differentiation into neural cells, and the effects of the three chemicals were evaluated by quantifying the expression levels of neural-specific proteins and measuring AChE activity.
Neural development is has been well characterized in rodents. During in vivo neurogenesis, the neuroectoderm differentiates into NPCs as precursor cells that subsequently commit to neural lineages such as neuronal and glial cells [22]. The in vitro differentiation of ESCs into neural cells mimics in vivo development. Therefore, cells undergoing in vitro differentiation can be useful as an alternative system for risk assessment to predict embryotoxic effects of chemicals and drugs [30,31]. The EST has been demonstrated to be a valuable tool for evaluating cytotoxicity with a reported accuracy of 78% [13]. However, a prerequisite for the use of EST for toxicity testing and risk assessment of chemicals and drugs is that the target cells and in vitro differentiation should be optimized.
During the in vitro transformation into neural cells, ESCs could develop into multiple types of neural cells expressing neural cell-specific markers as shown in Fig. 1. In our investigation, assessing developmental neurotoxicity of chemicals was done with whole populations of ESCs rather than pure subpopulations (i.e., Tuj1- or MAP2-positive cells) [2,30]. Therefore, our study provided an overview of chemical-induced neurotoxic effects observed during in vitro neural differentiation. It should be noted that the toxic effects could be differently induced depending on ESC line or species of origin, particularly mice compared to humans. To validate a mouse-based EST as an in vitro alternative model, further investigation of the variability of cell lines and reproducibility in human ESCs should be conducted.
To evaluate neurotoxic effects of test compounds on neural development, the non-cytotoxic concentrations (IC15) of each chemical were identified by an MTT assay in ESCs but not neural cells. According to our preliminary study, ESCs are generally less sensitive to chemicals than cell lines (e.g., embryonic germ cells, embryonic carcimoma cells, hepatoma, fibroblast, myeloma, and neuroblastoma cells) but the cytotoxicities of chemicals are dependent on cell type (data not shown). ESC-derived neural cells can be mixed with various cell types of neural lineage during the differentiation process. Therefore, the non-cytotoxic concentrations of each chemical determined by the IC15 of ESCs can elicit cytotoxic effects on some types of differentiated neural cells. To compensate for these drawbacks, we used the IC15 of each chemical as the highest concentrations of the test chemicals among seven different doses. The expression levels of each protein marker were corrected according to cell number.
Quantitative changes of different endpoints, including RNA and protein expression, may be useful indicators with which to assess cytotoxic effects induced by toxicants [15,30,31]. The kinetics of RNA expression for genes such as POU5F1, PAX6, NCAM1, Nestin, NEFL NEUROD1, MAP2, and Tuj1 during human neural precursor differentiation have been previously investigated [39]. Additionally, Stummann et al. [31] quantitatively measured the effects of neurotoxic chemicals on the mRNA expression of selected neural marker genes (MAP2, NCAM1, and NEUROD1). In the present study, we quantified the expression of several neural-specific proteins by measuring fluorescence signals following immunocytochemical labeling of the target factors. Proteins regulate almost all cellular functions. Compared to RNA, the quantitative analysis of appropriately selected proteins may therefore provide more definitive information about cellular alterations resulting from exposure to chemicals. However, the quantification of proteins requires specific controls to obtain precise results because the protein levels are measured indirectly as the fluorescence of secondary agents. Thus, the density of each marker protein was divided by the cell number in the present study to compensate for unspecific factors that might affect signal density.
POU5F1 is a transcription factor that regulates ESC self-renewal [23]. During ESC neural differentiation, POU5F1 levels gradually decrease as Nestin expression increases [28]. In the present study, the expression of Nestin increased gradually before reaching a maximum on Day 6. During this period, the expression of POU5F1 was slightly down-regulated and dropped significantly after Day 8. More importantly, the expression of POU5F1 was increased by exposure to all test chemical. This effect may be related to the chemically induced inhibition of neural differentiation associated with apoptosis and altered cell growth.
MM preferentially induces adverse effects in the central nervous system (CNS) because it penetrates the blood-brain barrier [1] and causes mitotic arrest by inhibiting microtubule formation [7]. This chemical produces different patterns of toxicity at various developmental stages according to exposure times and concentrations [25]. Three major mechanisms of cytotoxicity may be involved in the responses to MM exposure: 1) perturbation of intracellular calcium homeostasis [14], 2) induction of oxidative stress [26], and 3) inhibition of protein synthesis [6]. In our investigation, we observed that the levels of all neural proteins during the differentiating and differentiated stages were decreased by MM in a dose-dependent manner within a nanomolar range regardless of concentration (Fig. 4). However, Nestin expression during the differentiating stage and GABAA-R levels during the differentiated stage were more affected by lower doses of MM. These results suggest the involvement of secondary mechanisms. For instance, MM affects GABAergic synapses [4]. In addition, very low concentrations of MM inhibit Notch signaling that plays a critical role in neural stem cell differentiation [33]. Therefore, it is possible that disruption of the GABAergic system and Notch signaling may result in reduced expression of GABAA-R and Nestin, respectively.
Inorganic arsenic is a teratogenic compound that induces in the development of various malformations during embryogenesis [11,32]. Moreover, inorganic arsenic accumulates in the embryonic neuroepithelium [19], resulting in neural tube defects [37]. On the other hand, there is no evidence that organic arsenic (such as AA) has neurotoxic effects. However, we observed significant dose-dependent decreases in the expression of all neural markers during both stages in the current study. In particular, Nestin and Tuj1 levels during the differentiating stage along with GABAA-R and GFAP expression during the differentiated stage were significantly repressed. Based on our data, it is clear that that AA induces neurotoxicity by repressing the expression of neural-specific proteins rather than inducing cytotoxicity depending on concentration and exposure time. Further studies will be required to understand the mechanisms underlying the effects of AA on protein marker expression. In addition, additional animal studies will be needed to determine whether the in vitro effects of AA are reproducible in vivo.
DF is categorized as an FQ and used as a broad-spectrum antibacterial drug that interferes with DNA replication. In the present study, DF decreased the expression of all neural markers during both stages in a dose-dependent manner. The GABAA-R was less affected during the differentiating stage while the levels of this protein during the differentiated stage were significantly decreased by DF at concentrations greater than 10 µM. It is well known that quinolone acts as a GABA antagonist that blocks the ATP-dependent potassium channel [5,9]. This blockage might be one possible mechanism underlying the inhibition of neural marker expression induced by DF during the differentiated stage. However, it is difficult to accept that the down-regulation of neural markers including GABAA-R was caused by DF-induced neurotoxic effects. So far, FQs are known to very rarely induce adverse CNS effects. Additionally, there is no report showing that DF can penetrate the blood-brain barrier or placenta in vivo. Therefore, further study is needed to determine whether the concentrations of DF we used for our in vitro assay are relevant in vivo.
AChE, the enzyme responsible for acetylcholine degradation, is expressed in the embryonic nervous system [27]. AChE is a possible biomarker for evaluating the function of neural cells, and assays of this enzyme are widely used to diagnose Alzheimer's disease and neural defects [29,35]. In our study, an AChE assay was used to detect chemical toxicity during neural differentiation, and AChE activities were compared between the treated and control groups. As shown in Fig. 6A, AChE activities were significantly different between the controls of the differentiating and differentiated stages. These results suggest that differentiated neural cells generated larger quantities of acetylcholine than differentiating cells. When comparing the treated groups with their respective controls, the neurotoxic effects were increased in a dose-dependent manner and were likely to be more severe during the differentiating stage than the differentiated stage. This difference may have resulted from the lower basal AChE activity in the control for the differentiating stage. Further studies using phenotypically committed cell types like cholinergic neurons will confirm the usefulness of the AChE assay for assessing neurotoxicity.
In summary, MM, AA, and DF down-regulated the expression of neural-specific markers in a concentration-dependent manner during ESC neural differentiation. We tested the in vitro toxicity of these three chemicals and the effects on neural development by quantifying protein marker expression. In particular, the changes of neural-specific protein levels were evaluated in both developing and differentiated neural cells. Our results demonstrated that the in vitro neurotoxicity test based on ESC neural differentiation can be useful for assessing the impact of chemicals and drugs on neuronal development. However, further testing of positive and negative reference compounds is needs to validate the potential of our predictive model. Elucidation of the molecular mechanism underlying the inhibition of neural protein expression by these agents during ESC neural differentiation would also improve the utility of our in vitro model.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download