Abstract
Chronic enteritis can produce an excess of reactive oxygen species resulting in cellular damage. Stanniocalcin-1(STC-1) reportedly possesses anti-oxidative activity, the aim of this study was to define more clearly the direct contribution of STC-1 to anti-oxidative stress in cattle. In this study, primary intestinal epithelial cells (IECs) were exposed to hydrogen peroxide (H2O2) for different time intervals to mimic chronic enteritis-induced cellular damage. Prior to treatment with 200 µM H2O2, the cells were transfected with a recombinant plasmid for 48 h to over-express STC-1. Acridine orange/ethidium bromide (AO/EB) double staining and trypan blue exclusion assays were then performed to measure cell viability and apoptosis of the cells, respectively. The expression of STC-1 and apoptosis-related proteins in the cells was monitored by real-time PCR and Western blotting. The results indicated that both STC-1 mRNA and protein expression levels positively correlated with the duration of H2O2 treatment. H2O2 damaged the bovine IECs in a time-dependent manner, and this effect was attenuated by STC-1 over-expression. Furthermore, over-expression of STC-1 up-regulated Bcl-2 protein expression and slightly down-regulated caspase-3 production in the damaged cells. Findings from this study suggested that STC-1 plays a protective role in intestinal cells through an antioxidant mechanism.
Young calves are highly susceptible to infection by intestinal organisms. These pathogens are important causes of chronic enteritis - a disease associated with dyspepsia and diarrhea. The mechanism underlying this type of chronic inflammation remains largely unknown. Recent reports have linked the over-production of reactive oxygen species (ROS) to the development of chronic enteritis due to oxidative cellular damage [4,18].
It is often very difficult to diagnose chronic enteritis in neonatal calves by conventional methods. Current diagnostic techniques are limited to monitoring clinical symptoms and characteristic pathologic changes. Comparison of gene expression patterns for some characteristic cytokines like interleukin [16] in healthy animals and ones with chronic enteritis using molecular biological techniques has also been a reliable strategy. Recent studies have shown that STC-1, a hormone originally identified in bony fish [25] and then in mammals [5], suppresses superoxide generation [12], suggesting the involvement of STC-1 in anti-inflammation and anti-apoptosis activities. In some models of inflammation, increased expression of STC-1 relative to wild-type counterparts has been observed [22]. This finding may provide a new method for diagnosing chronic enteritis in calves by evaluating the alterations of STC-1 expression. STC-1 has also been utilized as a potential blood marker for predicting tumor aggressiveness [1], implying that the concentration of this molecullar in serum has important clinical significance. Additionally, the fact that STC-1 can suppress superoxide generation [12] might offer a new approach to limiting chronic enteritis-induced damage in neonates.
STC-1 is a secreted glycoprotein that functions in fish as a regulator of Ca2+/PO43- homeostasis [24]. In mammals, STC-1 is normally undetectable in the blood but ubiquitously expressed in several tissues, implying that this protein maybe act as a pleiotropic factor in a paracrine/autocrine manner [6]. However, STC-1 was recently identified in the blood of young calves starting at birth [23]. Therefore, the goal of our investigation was to assess the potential relationship between STC-1 and chronic enteritis.
This study used the primary intestinal epithelial cells (IECs) obtained from neonates was due to the in vitro proliferative potential of them was higher than that of commercially available IEC cell lines those are thought to be inadequate models of the intact epithelium because long-term growth in vitro requires morphological and biochemical changes.
The experimental protocol was approved by the official Committee on the Ethics of Animal Experiments of Huazhong Agricultural University (China).
The growth medium of IEC was a high-glucose formulation of Dulbecco's modified Eagle medium (DMEM; HyClone Laboratories, USA) supplemented with 5% fetal bovine serum (Hyclone Laboratories), 100 U/mL penicillin (Sigma, USA), 100 µg/mL streptomycin (Sigma), 5 µg/mL insulin (Sigma), 10 ng/mL epidermal growth factor (Sigma), 4 mM glutamine (Gibco BRL, USA), and 100 µg/mL porcine mucosal heparin (Sigma). Duodenum tissues and cultured cells were rinsed with Ca2+ and Mg2+-free phosphate buffered saline (PBS, pH 7.2~7.4) containing 8.00 g of NaCl, 0.20 g of KCl, 3.93 g of Na2HPO4.12H2O, and 0.20 g of KH2PO4 in 1 L of double distilled H2O.
Male 1-day-old Chinese Holstein calves (Kangli dairy farm, China) were anesthetized and euthanized with an electric shock apparatus (Qingdao ITO Food Machinery Manufacture, China) prior to nursing. Approximately 10 cm of duodenum was aseptically removed and eliminated the mesentery. The duodenum was then placed into a freezer bottle (Schott duran, Germany) containing chilled serum-free DMEM supplemented with penicillin (1,000 IU/mL) and streptomycin (1,000 µg/mL).
The IEC used in this study was cultured with an approach based on a previous report [10].
Viable epithelial cells were separated from contaminating fibroblasts in the primary cultures as previously described by Moyer [20]. Growth medium containing 25 U/mL collagenase I (Sigma) was used to inhibit the growth of the remaining fibroblasts as previously described [8]. After being subcultured for 3 days, the collagenase-supplemented medium was replaced with normal growth medium. The nuclei and cytoplasm of the cells were stained with H&E double staining kit (Boster Biological Technology, China) following the manufacturer's protocol. An inverted microscope (Olympus IX71; Olympus Corporation, Japan) and scanning electron microscopy (Hitachi SU8010; Hitachi High-Technologies Corporation, Japan) were used to investigated the morphology of cells in the primary cultures as previously described [14].
Immunocytochemical staining was performed to distinguish cells of epithelial origin from contaminating fibroblasts growing in the primary cultures. The purified primary cultured cells, Chinese V79 hamster lung fibroblasts (as a negative control; ATCC, USA), and rat IEC-6 intestinal crypt epithelial cells (as a positive control; ATCC) were seeded on sterile collagen-coated coverslips (Sail brand, China). The cells were incubated at 37℃ for 1 h with the mouse monoclonal antibodies directed against mouse vimentin (a type III intermediate filament protein specifically expressed in fibroblast), pan-cytokeratin (PCK, intermediary type II filaments specifically expressed in epithelia), and cytokeratin 18 (CK18, intermediary type I filaments specifically expressed in epithelia) [1 : 400 in PBST (0.5% Tween 20 in 0.02 M PBS); all three antibodies were purchased from Boster Biological Technology]. Primary antibody binding was visualized with biotinylated goat anti-mouse IgG (Abcam, USA) 1 : 8,000 dilution for 1 h at room temperature (RT) and a Vectastain ABC peroxidase detection system (Vector Laboratories, USA). An inverted microscope was used to examine the stained cells.
Total mRNA was obtained from bovine kidney using TRizol reagent (Invitrogen, USA) according to the manufacturer's instructions. A reverse transcription kit (Takara Bio, Japan) was used to synthesize cDNA. The full-length coding sequence of bovine STC-1 (Genebank Accession No. NM_176669.3) was amplified by PCR using the following primers: 5'-ATCAAGCTTATGCTCCAAAACTCAGCAG-3' (sense) and 5'- ATTCTAGACTAGGCACTCTCCTGGGAG-3' (anti-sense). Reactions consisted of 1× PCR buffer (containing 10 mM Tris-HCl, 50 mM KCl and 0.1% TritonX-100), 200 µM for each dNTP, 2 µg cDNA template, 1.5 mM MgCl2, 1.5 U Taq Plus DNA Polymerase (all of the PCR reagents were purchased from Takara Bio), and 0.2 µM forward and reverse primers (Sangon Biotech, China). After denaturation at 94℃ for 5 min, the reaction proceeded for 30 cycles of 94℃ for 15 sec, 60℃ for 15 sec and 72℃ for 25 sec. And finally extended for 5 min at 72℃. The amplicon was cloned into a pcDNA3.1+ plasmid (Invitrogen) at the HindIII and XbaI (Takara Bio) sites. The recombinant plasmid was named pcDNA/ STC-1. The pcDNA/STC-1 plasmid was then transformed into DH5α cells (Invitrogen) and the cells were cultured in Lysogeny broth medium at 37℃ overnight.
A commercial kit (Omega Bio-Tek, USA) was used to extract the endotoxin-free plasmid from overnight cultures of transformed DH5α cells. The primary IECs were transfected with pcDNA/STC-1 (4.0 µg/well), an equivalent amount of pcDNA3.1+ vector, or an equivalent volume of PBS in lieu of plasmid (as control) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Total mRNA and protein were harvested from the cells at 0, 24, 48, and 72 h post-transfection.
To mimic damage associated with chronic enteritis, the IECs were incubated at 37℃ and treated with or without 200 µM H2O2 in growth medium for different periods of time (4, 8, 12, 16, and 24 h).
To determine the effect of STC-1 on H2O2-induced damage, the IECs were divided into six groups. Cells in the first group were incubated at 37℃ in growth medium without H2O2 for 56 h (as control group). IECs in the second group were cultured for 48 h in growth medium and then treated with 200 µM H2O2 for 8 h. Cells in the third and fourth groups were transfected with pcDNA/STC-1 or pcDNA3.1+, respectively, and then cultured in growth medium for 56 h. Finally, cells in the fifth and sixth groups were transfected with pcDNA/STC-1 or pcDNA3.1+, respectively; cultured in growth medium for 48 h, and then treated with 200 µM H2O2 for 8 h.
Total mRNA and proteins were harvested from a portion of the treated cells, and the other treated cells were detached with 0.04% (w/v) trypsin (Sigma) for a cell viability test.
Viability of the IECs treated with or without H2O2 was evaluated by double staining with AO/EB (Sigma). After removing the H2O2, the cells were washed with PBS and trypsinized. A mixture containing 100 µL of the cell suspension containing about 105 cells/mL in PBS and 10 µL of the AO/EB working solution (50 µg/mL of AO and 50 µg/mL of EB in PBS) was then prepared. The cells were immediately analyzed with a fluorescence microscope (Olympus IX71; Olympus Corporation). Cell death rate was monitored by trypan blue exclusion with a hemocytometer (Nexcelom Bioscience, USA) as previously described [19].
Total RNA extraction and cDNA preparation were performed for each sample in the manner detailed above. Amplification and quantification were performed by a StepOne Real-Time PCR System (Applied Biosystems, USA) with SYBR Green I. Sequences of the primers for STC-1 were 5'-AGTGATTCGCTGCCTCAACA-3' (sense) and 5'-TCTCCAGGCATGCAAAAGCT-3' (anti-sense). Sequences of the primers for GAPDH (as a control) were 5'-CCTTCATTGACCTTCACTACATGGTCTA-3' (sense) and 5'-TGGAAGATGGTGATGGCCTTTCCATTG-3' (anti-sense). Reactions consisted of 1 × SYBR Green I (Takara Bio), 0.2 µM forward and reverse primers (Sangon Biotech) for each gene, and 1/50 of total reaction volume of ROX. The thermal cycling conditions were 5 min at 94℃ followed by 40 cycles of 20 sec at 94℃, 20 sec at 60℃ for each gene, and 15 sec at 72℃ with a final 10 min extension at 72℃.
Cells were lysed for 10 min with ice-cold lysis kit (Beyotime Institute of Biotechnology, China) supplemented with a protease inhibitor mixture (Sigma). An 8-min centrifugation was performed at 12,000 × g to collect the supernatant whose protein concentration was measured using a BCA Protein Assay kit (Beyotime Institute of Biotechnology). A total of 30 µg of proteins from each sample were separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (EMD Millipore, USA). The membranes were then blocked with 5% (w/v) non-fat milk (CWbio, China) in PBST for 2 h at RT. Next, the blots were incubated with rabbit polyclonal antibodies against human STC (1 : 500 in PBST; Abcam), Bcl-2 (1 : 600; Sangon Biotech), Bax (1 : 600; Sangon Biotech), caspase-3 (1 : 600; ABclonal Biotechnology, China), and GAPDH (1 : 5,000; Abcam) at RT for 2 h. The membranes were subsequently washed six times with PBST for 5 min at RT and then incubated with goat anti-rabbit IgG-HRP for 2 h at RT (1 : 8,000; CWbio). The immunoreactive bands were visualized using an Enhanced chemiluminescence (ECL) kit (CWbio).
The best procedure for isolating IECs was formulated based on our preliminary experiments showing that digestion with collagenase I for approximately 2 h yields high quality individual crypts or villi (panel A in Fig. 1) from intestinal epithelium. When adhered to the flask, the purified individual villi gave rise to proliferating foci of cells (panel B in Fig. 1) that formed confluent monolayers within 3~7 days. After detachment with 0.04% trypsin, reattachment, and purification, the IECs formed a homogeneous pavement-like layer typical of epithelial sheets (panel C in Fig. 1). The cells had a dome-like morphology as seen with the differential interference contrast microscope (panel D in Fig. 1), and a globose morphology with a rough surface when viewed with the scanning electron microscope (panel E in Fig. 1). The nuclei and cytoplasm of the cells stained with H&E were blue and pink, respectively (panel F in Fig. 1).
Results of the immunocytochemical staining experiment indicated that the primary IECs and IEC-6 cells exhibited strong immunoreactivity specific for PCK and CK18 (panels A1, A2, C1, and C2 in Fig. 2) but not vimentin (panels A and C in Fig. 2). In contrast, only yellow staining corresponding to vimentin was found in the V79 cells (panel B in Fig. 2); immunoreactivity against PCK or CK18 was not observed (panels B1 and B2 in Fig. 2). These results suggest that epithelial cells were the major component of the primary cultures.
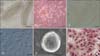
The expression of STC-1 was evaluated by real-time PCR and Western blotting. We found that transfection with pcDNA/STC-1 was effective for over-expressing STC-1. In cells containing this construct, mRNA expression levels in the cells were maximized at approximately 48 h after transfection (p < 0.001, panel A in Fig. 3) whereas protein expression was maximized at 72 h (panel B in Fig. 3). On the other hand, no significant changes in either STC-1 mNRA or protein expression were observed in cells transfected with the pcDNA3.1+ vector or the PBS blank (panels A and C in Fig. 3). Ultimately, the cells were used for further study 48 h after transfection considering the expression patterns of STC-1 and cell growth.
Cell viability (panels A-A5 in Fig. 4) and the cell survival rate (p < 0.001, panel B in Fig. 4) decreased in a time-dependent manner when the cells were exposed to 200 µM H2O2 as measured by AO/EB double staining and the trypan blue exclusion assay, respectively. A time-dependent increase in STC-1 mRNA and protein expression was also observed in the cells with H2O2-induced damage (p < 0.01, panels C and D in Fig. 4), suggesting that STC-1 expression can be stimulated by oxidative stress. Decreased cell viability and survival rates resulting from H2O2 treatment were significantly improved by pre-transfection with pcDNA/STC-1 (panel A2 and the fifth column in panel B in Fig. 5, p < 0.01). Protection against damage by H2O2 was not observed in the cells pre-transfected with the pcDNA3.1+ vector (panel A3 and sixth column in panel B in Fig. 5, p < 0.1).
We also found that the expression of Bcl-2 protein was significantly down-regulated in the cells treated with H2O2 alone for 8 h (panel C in Fig. 5) or H2O2 for 8 h after pcDNA3.1+ pre-transfection for 48 h (Lane 3 in panel C in Fig. 5) compared to the control group (Lane 1 in panel C in Fig. 5). Pre-transfection with pcDNA/STC-1 restored Bcl-2 expression (panel C in Fig. 5). Interestingly, the expression patterns of caspase 3 protein were opposite to those observed for Bcl-2. Additionally, no change in the expression of Bax protein in the H22O2 treatment groups was observed (panel C in Fig. 5) although expression was enhanced compared to that found in the control group (panel C in Fig. 5).
The major goal of our study was to elucidate the relationship between STC-1 and chronic enteritis. This investigation was restricted by a lack of adequate diseased animals and difficulties associated with making in vivo observations. Therefore, cultured primary IECs were harvested and used for our investigation. To mimic cellular damage induced by chronic enteritis, the primary cells were treated with H2O2 for 4 ~ 24 h. This strategy helped ensure the continuity of reactive oxygen species (ROS) production found during chronic inflammation, and was based on previous work by Bae et al. [2] and our group [28] who treated MCF-7 cells or cardiomyocytes for 24 h with 200 µM H22O2 to induce apoptosis.
As reported in the literature, chronic enteritis is a common disease of several mammalian species caused by multiple factors [26]. The lack of convenient animal models and complexity of diagnosing this condition made prior studies in cattle somewhat sporadic. In previous investigations, oxidative stress was thought to be one mechanism underlying this inflammatory disorder [9] since excessive endogenous ROS produced during chronic enteritis inevitably lead to the activation of critical cellular survival pathways in IECs [27]. Theoretically, the prevention of ROS over-production and increased ROS scavenging could be an effective strategy for decreasing cell injury. STC-1 has been found to suppress H2O2 production [11] and promote H2O2 decomposition [3], indicating that this factor exerts an antioxidant effect in the gut during oxidative injury. Consistent with these findings, our results clearly demonstrated that STC-1 over-expression increased cell viability and attenuated the injury of bovine IECs that were exposed to H2O2. These effects may be related to the abilities of STC-1 to uncouple oxidative phosphorylation in mitochondria [11,22] and modulate the activities of some enzymes that scavenge H2O2 [3]. Our results suggest that increasing STC-1 expression might help treat chronic enteritis and prevent enterocyte apoptosis promoted by oxidative stress.
Given the complexity of chronic enteritis pathogenesis, an accurate diagnosis is very difficult to make. Monitoring the cytokines involved in inflammation could be a promising direction. For example, interleukin is a well-characterized indicator of an inflammatory response and has been widely used to diagnose various types of inflammation [13]. Previous studies demonstrated that STC-1 is normally absent from the blood [6,7]. However, recent research determined that this factor exists in the blood of calves starting at birth [23]. Therefore, measuring the level of STC-1 in blood might be a novel way to predict the development of chronic inflammatory disease. Our data indicated that the expression of STC-1 was induced by H2O2 in a time-dependent manner. Thus, the level of expression was positively associated with the extent of H2O2-induced cellular damage. These results suggest that the induction of STC-1 expression might be a general adaptive and protective response to oxidative stress, and support an earlier finding that STC-1 gene expression is stimulated by oxidative stress [21]. It is still unclear how STC-1 is up-regulated by oxidative stress and the exact mechanism underlying its protective role is still unknown. Yeung et al. [29] reported that the expression of STC-1 is stimulated by hypoxia inducible factor-1 (HIF-1). Law et al. [15] identified a putative hypoxia response element (HRE) site in the STC1 gene, suggesting that STC-1 expression is controlled by a complicated regulatory mechanism. Further study is needed to determine whether HIF-1 mediates the up-regulation of STC-1 in the presence of H2O2.
Our current investigation had several limitations. We lacked an appropriate animal model or access to animals suffering from chronic enteritis. These were major weaknesses. However, all of these problems will be addressed in future studies.
In conclusion, we determined that altered expression of apoptosis-related proteins is an effect of H2O2-induced oxidative injury. We also found that the expression of Bcl-2, a well-characterized anti-apoptotic protein, was significantly enhanced by H2O2 treatment and STC-1 over-expression compared to exposure to H2O2 alone. H2O2 and STC-1 generation had the opposite effect on caspase 3 expression. The findings suggested that the ability of STC-1 to attenuate H2O2-induced damage is partly due to up-regulated Bcl-2 expression [17].
Figures and Tables
 | Fig. 1Morphology of intestinal cells in culture. Phase contrast microscopy was performed to observed characteristics associated with the evolution of bovine IECs in primary cultures starting from adherent organoid structures (A) that gave rise to lager proliferating foci (B) which joined together to form a confluent monolayer. After purification, the subcultured epithelial cells grew dispersedly in the flask (C). The individual epithelial cell had a dome-like and rounded morphology when viewed with a differential interference contrast microscope (D) or scanning electron microscope (E), respectively. The nuclei and cytoplasm of the hematoxylin- and eosin-stained cells were blue and pink, respectively (F). ×40 (A), ×200 (B ~ D, and F), and ×15,000 (E). Scale bars = 200 µm (A) or 50 µm (B ~ F). |
 | Fig. 2Characterization of cells stained for immunocytochemistry. Panels show the primary cultures, V79, and IEC-6 cells, and indicate cells stained with antibodies against vimentin, PCK, CK18, or PBS (as the negative control), from left to right, respectively. Note that both the primary cultures (A1 and 2) and IEC-6 cells (C1 and 2) were stained yellow by the antibodies against PCK and CK18, but not by the anti-vimentin antibody (A and C). In contrast, the V79 cells displayed opposite patterns of immunoreactivity (B ~ B2). ×400 (A1 and 2), ×200 (A, A3, B ~ B3, and C ~ C3). Scale bars = 20 µm (A1 and 2) or 50 µm (A, A3, B ~ B3, and C ~ C3). |
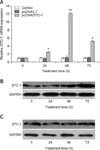 | Fig. 3Analysis of STC-1 mRNA and protein expression in the transfected IECs. The expression of STC-1 mNRA in cells transfected with the pcDNA/STC-1 construct was maximized approximately 48 h after transfection as measured by real-time PCR (gray column in A; *p < 0.01 or **p < 0.001 vs. the control cells). Protein expression was maximized at 72 h as measured by Western blotting (B). In contrast, no significant changes were observed in STC-1 mRNA or protein expression in cells transfected with the pcDNA3.1+ vector (black column in A and C). |
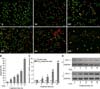 | Fig. 4Detection of viability, apoptosis, and STC-1 expression of transfected IECs. A time-dependent decrease in cell viability (A ~ A5, cells treated with H2O2 for 0, 4, 8, 12, 16, and 24 h) and survival rate (B, *p < 0.001 vs. 0 h) was observed for cells treated with H2O2 using AO/EB double staining and a trypan blue exclusion assay, respectively. Additionally, time-dependent increases in STC-1 mRNA (C, #p < 0.01 or ##p < 0.001 vs. the control cells) and protein expression (D, C: control cells, T: H2O2-treated cells) were found in the cells exposed to 200 µM H2O2 using real-time PCR and Western blotting, respectively. ×400 (A ~ A5). Scale bars = 20 µm (A ~ A5). |
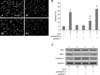 | Fig. 5The effects of STC-1 over-expression on the viability, apoptosis rate, and apoptosis-related protein expression in IECs. (A ~ A3) Viability of cells grown normally, treated with H2O2 for 8 h, or pre-transfected with pcDNA/STC-1 or pcDNA3.1+ for 48 h before H2O2 treatment, respectively. In cells that over-overexpressed STC-1, H2O2-induced cellular injury was significantly attenuated as measured by AO/EB double staining (A3). (B) A significant decrease of apoptosis induced by H2O2 was observed in cells transfected with pcDNA/STC-1 (fifth column) as measured by a trypan blue exclusion assay (#p < 0.01 vs. the control; ap < 0.01 vs. cells treated with H2O2 alone; bp < 0.01 vs. the pcDNA3.1+-transfected cells treated with H2O2). (C) Expression of apoptosis-related factors measured by Western blotting. Lane 1, Cells grown normally as the control; Lane 2, cells treated with H2O2 alone for 8 h; Lane 3, cells treated with H2O2 alone for 8 h after transfection with pcDNA3.1+ for 48 h; Lane 4, cells treated with H2O2 alone for 8 h after transfection with pcDNA/STC-1 for 48 h. Cells transfected with pcDNA/STC-1 showed a significant up-regulation of Bcl-2 expression and a slight down-regulation of caspase 3 expression. ×400 (A1~A4). Scale bars = 20 µm (A1 ~ A4). |
Acknowledgments
This work was supported by the National Natural Sciences Foundation of China (No. 31172374), the National Key Technology R&D Program (2012BAD12B03-1-5), and the Opening Project of the Hubei Key Laboratory of Animal Embryo and Molecular Breeding at the Hubei Academy of Agricultural Science (2013ZD120).
References
1. Arigami T, Uenosono Y, Ishigami S, Hagihara T, Haraguchi N, Matsushita D, Yanagita S, Nakajo A, Okumura H, Hokita S, Natsugoe S. Expression of stanniocalcin 1 as a potential biomarker of gastric cancer. Oncology. 2012; 83:158–164.

2. Bae JY, Ahn SJ, Han W, Noh DY. Peroxiredoxin I and II inhibit H2O2-induced cell death in MCF-7 cell lines. J Cell Biochem. 2007; 101:1038–1045.

3. Baioni L, Basini G, Bussolati S, Grasselli F. Stanniocalcin 1 affects redox status of swine granulosa cells. Regul Pept. 2011; 168:45–49.

4. Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg. 2006; 391:499–510.

5. Chang ACM, Janosi J, Hulsbeek M, de Jong D, Jeffrey KJ, Noble JR, Reddel RR. A novel human cDNA highly homologous to the fish hormone stanniocalcin. Mol Cell Endocrinol. 1995; 112:241–247.

6. De Niu P, Radman DP, Jaworski EM, Deol H, Gentz R, Su J, Olsen HS, Wagner GF. Development of a human stanniocalcin radioimmunoassay: serum and tissue hormone levels and pharmacokinetics in the rat. Mol Cell Endocrinol. 2000; 162:131–144.

7. Deol H, Stasko SE, De Niu P, James KA, Wagner GF. Post-natal ontogeny of stanniocalcin gene expression in rodent kidney and regulation by dietary calcium and phosphate. Kidney Int. 2001; 60:2142–2152.

8. Föllmann W, Weber S, Birkner S. Primary cell cultures of bovine colon epithelium: isolation and cell culture of colonocytes. Toxicol In Vitro. 2000; 14:435–445.

9. Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997; 2:d12–d26.

10. Freshney RI, Freshney MG. Culture of Epithelial Cells. 2nd ed. New York: Wiley-Liss;2004. p. 303–336.
11. Huang L, Belousova T, Chen M, DiMattia G, Liu D, Sheikh-Hamad D. Overexpression of stanniocalcin-1 inhibits reactive oxygen species and renal ischemia/reperfusion injury in mice. Kidney Int. 2012; 82:867–877.

12. Huang L, Garcia G, Lou Y, Zhou Q, Truong LD, DiMattia G, Lan XR, Lan HY, Wang Y, Sheikh-Hamad D. Anti-inflammatory and renal protective actions of stanniocalcin-1 in a model of anti-glomerular basement membrane glomerulonephritis. Am J Pathol. 2009; 174:1368–1378.

13. Kopa Z, Wenzel J, Papp GK, Haidl G. Role of granulocyte elastase and interleukin-6 in the diagnosis of male genital tract inflammation. Andrologia. 2005; 37:188–194.

14. La Ragione RM, Cooley WA, Woodward MJ. The role of fimbriae and flagella in the adherence of avian strains of Escherichia coli O78:K80 to tissue culture cells and tracheal and gut explants. J Med Microbiol. 2000; 49:327–338.

15. Law AYS, Ching LY, Lai KP, Wong CKC. Identification and characterization of the hypoxia-responsive element in human stanniocalcin-1 gene. Mol Cell Endocrinol. 2010; 314:118–127.

16. Lee H, Stabel J, Kehrli ME Jr. Cytokine gene expression in ileal tissues of cattle infected with Mycobacterium paratuberculosis. Vet Immunol Immunopathol. 2001; 82:73–85.

17. Liu G, Yang G, Chang B, Mercado-Uribe I, Huang M, Zheng J, Bast RC, Lin SH, Liu J. Stanniocalcin 1 and ovarian tumorigenesis. J Natl Cancer Inst. 2010; 102:812–827.

18. Lykkesfeldt J, Svendsen O. Oxidants and antioxidants in disease: oxidative stress in farm animals. Vet J. 2007; 173:502–511.

19. McGill G, Shimamura A, Bates RC, Savage RE, Fisher DE. Loss of matrix adhesion triggers rapid transformationselective apoptosis in fibroblasts. J Cell Biol. 1997; 138:901–911.

20. Moyer MP, Aust JB. Human colon cells: culture and in vitro transformation. Science. 1984; 224:1445–1447.

21. Serlachius M, Andersson LC. Upregulated expression of stanniocalcin-1 during adipogenesis. Exp Cell Res. 2004; 296:256–264.

22. Sheikh-Hamad D. Mammalian stanniocalcin-1 activates mitochondrial antioxidant pathways: new paradigms for regulation of macrophages and endothelium. Am J Physiol Renal Physiol. 2010; 298:F248–F254.

23. Tremblay G, Delbecchi L, Loiselle MC, Ster C, Wagner GF, Talbot BG, Lacasse P. Serum levels of stanniocalcin-1 in Holstein heifers and cows. Domest Anim Endocrinol. 2009; 36:105–109.

24. Wagner GF, Jaworski E. Calcium regulates stanniocalcin mRNA levels in primary cultured rainbow trout corpuscles of stannius. Mol Cell Endocrinol. 1994; 99:315–322.

25. Wagner GF, Hampong M, Park CM, Copp DH. Purification, characterization, and bioassay of teleocalcin, a glycoprotein from salmon corpuscles of Stannius. Gen Comp Endocrinol. 1986; 63:481–491.

26. Watson PR, Galyov EE, Paulin SM, Jones PW, Wallis TS. Mutation of invH, but Not stn, reduces Salmonella-induced enteritis in cattle. Infect immun. 1998; 66:1432–1438.

27. Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996; 313:17–29.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download