Abstract
Figures and Tables
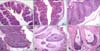 | Fig. 1Haematoxylin and eosin staining of (A) the bursa of Fabricius on E24. The star (⋆) indicates a lymphoid follicle with indistinct medulla and cortex. (B) The bursa of Fabricius on E27. (C) The bursa of Fabricius on P20. (D) The bursa of Fabricius on P70. (E and F) The bursa of Fabricius on P200. Asterisks (*) indicate large mucoid cysts in the lymphoid follicles. FAE: follicle-associated epithelium, IFE: interfollicular epithelium. C, lymphoid follicle cortex; M, lymphoid follicle medulla. Scale bars = 1,000 µm (A~F). |
 | Fig. 2DNA histogram of the bursa cell cycle obtained by flow cytometry. Panels A-E correspond to E24, E27, P20, P70, and P200, respectively. |
 | Fig. 3Scattergram of apoptotic bursa cells obtained by flow cytometry. Panels A-E correspond to E24, E27, P20, P70, and P200, respectively. |
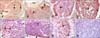 | Fig. 4Proliferation cell nuclear antigen (PCNA) assay results with hematoxylin counterstaining. (A) PCNA-positive reaction in the follicular cortex and medulla as well as the IFE on E24 (arrow); the FAE was PCNA-negative. (B) PCNA-positive reaction in the follicular cortex and medulla along with IFE on P20 (arrow); FAE was PCNA-negative. (C) PCNA-positive reaction in the follicular cortex and medulla on E24 (arrow). (D) PCNA-positive reaction in the follicular cortex and medulla on E27 (arrow). (E) PCNA-positive reaction in the follicular medulla on P20 (arrow). (F) PCNA-positive reaction in the follicular cortex and medulla on P70 (arrow). (G) PCNA-positive reaction in the follicular cortex and medulla on P200 (arrow). (H) Negative control for the follicle lymphocytes on E27. Scale bars = 20 µm (A and B), 10 µm (C~H). |
 | Fig. 5Transferase-mediated dUTP nick-end labeling (TUNEL) assay findings with haematoxylin counterstaining. (A) TUNEL-positive follicular lymphocytes containing free apoptotic bodies or nuclei with condensed chromatin (arrow with a longer tail), and apoptotic bodies in the cytoplasm of macrophages (arrow with a shorter tail) in the lymphoid follicle on P200. (B) TUNEL-positive follicular cortex and medulla as well as FAE on E24 (arrow). (C) TUNEL-positive follicular cortex and medulla on E27 (arrow); the IFE and FAE were negative. (D) TUNEL-positive follicular cortex and medulla, and FAE on P20 (arrow). (E) TUNEL-positive follicular cortex and medulla on P70 (arrow). (F) TUNEL-positive follicular cortex and medulla on P200 (arrow). (G) TUNEL-positive mucosa epithelium on P200 (arrow). (H) Negative control follicle lymphocytes and mucosa epithelium on P20. Scale bars = 10 µm (A), 20 µm (B~G), 40 µm (H). |
Table 2

Table 3

Figures in the columns marked with the same lowercase superscript letters are not significantly different (p > 0.05) while those marked with different lowercase superscript letters are significantly different according to age (p < 0.05). Different capital superscript letters (A, B) indicate significant differences in the proliferative lymphocyte density between the follicular cortex and medulla in the same age group (p > 0.05). On E24, distinct medulla and cortex were observed in only some lymphoid follicles. Asterisks (*) indicate that the data are estimated for lymphoid follicles with a distinct medulla and cortex. TL: total lymphocytes, PL: positive lymphocytes, PLD: proliferative lymphocyte density.
Table 4

Figures in columns marked with the same lowercase superscript letters are not significantly different (p > 0.05) while those marked with the different lowercase superscript letters are significantly different according to age (p < 0.05). Different capital superscript letters (A, B) indicate significant differences in the apoptotic lymphocyte density between the follicular cortex and medulla in the same age group. On E24, only some lymphoid follicles were found to have a distinct medulla and cortex. Asterisks (*) indicate that the data were estimated for lymphoid follicles with a distinct medulla and cortex. ALD: apoptotic lymphocyte density.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



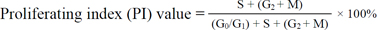
 XML Download
XML Download