Introduction
The performance level of athletic horses is determined by interdependent biological and physiological processes. Among these factors, hematological adaptation, such as changes in clotting times, is necessary to provide an appropriate blood supply during exercise. Several studies have examined the relationship between blood clotting times and exercise in both human and equine athletes [7,9,13,14,15,19]. Exercise has been shown to affect blood coagulation [8] by inducing an acute but transient increase in blood coagulability [11]. This change seems to be counterbalanced by a simultaneous increase in fibrinolytic activity that depends on both the intensity and duration of exercise [11,14]. In particular, it has been documented that fibrinolysis directly correlates with exercise intensity in human athletes [6].
In equines, conflicting observations have been made. Some researchers found evidence of increased fibrinogen degradation associated with exercise [10] whereas others failed to see changes in clotting parameters with physical activity [1,2]. These controversial results might be due differences in the intensity (maximal or sub-maximal effort), duration, and type (gallop, dressage, show jumping, or endurance) of exercise and training; sampling time (before, during, or after exercise), and assay methodologies [3,11,13,14,18]. Equivocal data reported in the literature also suggests a possible impact of physical conditioning on the hemostatic process similar to that found in humans [18]. Nevertheless, very few data about the effect of training on the hemostatic system in humans [15] and horses [14] have been published. Understanding how different kinds of exercise affect clotting parameters might be useful in order to develop advanced training programs for equine athletes. Therefore, the purpose of this study was to investigate the effect of training on changed certain with some clotting parameters such as protrombin time (PT), activated partial tromboplastin time (aPTT), and fibrinogen (Fb) in horses.
Materials and Methods
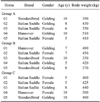
Fifteen clinically healthy horses from the same training centre located in Sicily (Italy) were enrolled in the present study. Individual characteristics (breed, gender, age, and body weight) of the horses are shown in Table 1. The diet consisted of hay (first cut meadow hay, sun cured, late cut; 8 ± 1 kg/day; 6.9 crude protein on average) and mixed cereals (oats and barley, 50% each, 3.5 ± 0.5 kg/day), three times a day (at 7:00, 12:00 and 18:00). Cereal mixture composition (dry matter basis) was 13.0% crude protein, 20.7% crude fiber, and 3.4% other extracts; the estimated net energy content was 0.8 UFC (Unitè Fouragire Cheval). Water was available ad libitum.
The horses were divided into three equal groups according to the training program (Table 2). Group A included horses that participated in the same daily training program they were accustomed to for the entire duration of the study: warm-up (10 min walk, 30 min trot, and 10 min gallop) and a show jumping course consisting of 10 fences with an average height of 100 ± 10 cm. Group B trained twice a week during the first 5 weeks of the study: warm-up (10 min walk, 20 min trot, and 10 min gallop) and a show jumping course including seven fences with an average height of 80 ± 10 cm. Afterwards, group B was subjected to a higher-level training program for an additional 5 weeks (group B1): warm-up (10 min walk, 30 min trot, and 10 min gallop) and a show jumping course with 10 fences (average height of 100 ± 10 cm). Group C included sedentary animals that were not trained during the study.
Blood samples were collected by jugular venipuncture into 3.6 mL vacutainer tubes containing 3.8% sodium citrate (Terumo Corporation, Japan) when the horses were at rest before feeding (at 6:00 am). During the first week, three samples were collected on the 1st, 3rd, and 5th days. Afterwards, blood samples were collected once a week. Samples were analyzed within 2 h after collection. Whole blood was centrifuged at 968 × g for 15 min, at room temperature. PT, aPTT, and Fb were assessed using standard kits for a Clot 2 coagulometer (SEAC diagnostics, Italy). The PT kit was based on the principle that addition of an adequately calcified amount of tissue factor (factor III) to citrated plasma activates factor VII, which induced the formation of a stable plug. The assay was performed by placing 200 mL of tissue factor (PT reagent) in a test tube preheated to 37℃ and subsequently adding 100 mL of citrated plasma. Upon the addition of the test plasma, the clotting time was measured with a stopwatch of the coagulometer (Clot 2; SEAC diagnostics). The time in seconds from plasma-reagent mixing to visible clot formation was defined as the PT.
The aPTT kit involved the addition of a platelet substitute (phospholipids and ellagic acid as a soluble activator) and calcium chloride, which induced the formation of a stable plug. The assay was performed by placing 100 mL of citrated plasma and 100 mL of aPTT reagent (preheated to 37℃) in a test tube preheated to 37℃. The solution was incubated for 3 min at 37℃, and 100 mL of calcium chloride preheated to 37℃ was then added. Upon the addition of calcium chloride, the clotting time was measured with a stopwatch. The time in seconds from calcium chloride addition to visible clot formation was defined as the aPTT.
The standard kit for Fb quantitation was based on the addition of a relatively large amount of thrombin to diluted citrated plasma, thereby ensuring that the clotting time depended only on the Fb contained in the sample. The assay consisted of placing 200 mL of diluted plasma (diluted 1 : 10 by combining 100 mL of plasma with 900 mL of buffer) in a test tube preheated to 37℃. The solution was incubated for 2 min at 37℃ before 100 mL of fibrinogen reagent was added. Upon the addition of the fibrinogen reagent, the clotting time was measured with a stopwatch. The time (seconds) required for clot formation was automatically converted into mg/dL by an automated mechanical endpoint coagulation instrument (Clot 2, SEAC diagnostics).
Recorded values were tested for normality using the Kolmogorov-Smirnov test. The p values < 0.05 were considered statistically significant. A two-way analysis of variance (ANOVA) for repeated measures was used to identify statistically significant effects of training time and type of training programs on PT, aPTT, and Fb values. All calculations were performed using the PRISM package (GraphPad Software, USA). Bonferroni's post hoc comparison was also performed for PT, aPTT, and Fb values. All animal treatments, housing, and care described above were carried out in accordance with the standards recommended by the European Union Directive 2010/63/EU for animal experiments.
Results
A two-way ANOVA for repeated measures revealed that training had significant effects (p < 0.05) on Fb plasma levels in group B1 alone. Plasma Fb levels increased during the first week after a more intense training program was started (Fig. 1A). During the same week, PT (Fig. 1B) and aPTT (Fig. 1C) were unchanged. None of parameters we studied were significantly altered during the entire experimental period (Fig. 2).
Discussion
The increased Fb concentration we observed in group B1 after intensifying the training program might indicate an adjustment to the new workload [5]. In fact, Fb changes recorded in the present study are within the reference range (100~400 mg/dL) [17], and so do not represent any pathological condition [16]. However, several authors [5,14,15,18] found different trends in Fb concentrations relative to training in both horse and human athletes. Our previous research [14] showed that fibrinolytic activity is enhanced in Standardbred horses after a specific training program for 1,600- and 2,000-meter trot races. Another study by Fazio et al. [5] demonstrated that Fb levels in Thoroughbreds increase during the first 20 days of the standard training program for these horses, and then return to baseline values after 80 days of training. In the current study, Fb changes recorded during our training program for jumpers were similar to trends observed by Fazio et al. [5] who suggested that the return of Fb concentrations to baseline levels represents an adaptive response to training. The same trend in fibrinogen concentrations was previously described in human athletes by Montgomery et al. [12] who speculated that this effect may have a genetic basis. However, Van den Burg et al. [18] did not observed any effect of training on fibrinolytic activity measured in men when resting while Prisco et al. [15] determined that fibrinolytic activity at rest decreased when untrained men followed a training program for several weeks.
Wang [19] did not observe significant differences in human PT levels or aPTT assessed at rest when evaluating sedentary subjects, joggers, and marathon runners. We also failed to identify differences in PT and aPTT resting levels among groups A, B, C, and B1. As previously mentioned, difference in data reported in the literature might be caused by variations in blood sampling techniques (clean venipuncture versus the use of a catheter that can provoke additional activation of clotting cascade) or the type (intensity and duration) of training that could affect the outcome of the study. Therefore, meticulous standardization of laboratory procedures should be required when studying hemostatic variables.
Changes in clotting parameters during physical activity may indicate the functional adaptation of an organism to exercise [15]. However, increased clotting activity in both human and equine athletes could represent the development of critical circulatory disorders such as disseminated intravascular coagulation. Therefore, monitoring hemocoagulative factors in athletic horses should provide useful information about the fitness level of the animals, especially during training programs and competitions [4,13].
Our findings showed that Fb is a parameter affected by physical training. During the first week of training, plasma Fb levels tended to increase. After this time, the Fb concentrations in group B1 decreased to baseline levels, suggesting that the horses had adapted to the new training program although changes in Fb levels never exceeded the physiological range. A better knowledge of haemostatic profile of sport horses might be useful to assess the performance level and adjust horse training schedules. However, further studies should be carried out to better elucidate the relationship between clotting parameters and training in athletic horses.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


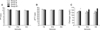
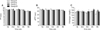

 XML Download
XML Download