Abstract
This report describes the gross, histological, and immunohistochemical features of medullary thyroid carcinoma (MTC) with pulmonary metastases in a young dog. Sheets of pleomorphic cells supported by fibrous stroma characterized the primary mass, while metastatic nodules had a neuroendocrine pattern. Despite differing histologic features, all masses showed marked immunoreactivity against calcitonin and multiple neuroendocrine markers consistent with MTC. Although MTC is a well-recognized entity, it may be difficult to distinguish this mass from other thyroid neoplasms, necessitating immunohistochemical characterization.
Thyroid neoplasms have a low prevalence (1~2%) [1]. While benign tumors of the thyroid are clinically underdiagnosed, carcinomas are locally aggressive and often metastasize to local lymph nodes and lungs. Neoplasms may arise from follicular epithelium or medullary C-cells, and the histological pattern may vary between follicular, compact, papillary, and mixed types [10]. Medullary thyroid carcinoma (MTC) accounts for less than 5% of thyroid neoplasms in dogs; accordingly, there have been only a few reports relative to other thyroid neoplasms [1,9,13,17,18]. However, this low prevalence might be due to misdiagnosis caused by their histological similarity with compact thyroid carcinoma [13,14,16,17]. Several studies in veterinary and human medicine have demonstrated that MTC differs immunohistochemically from other thyroid neoplasms [17,18], which can aid in its diagnosis. This report documents different histological and immunohistochemical patterns in a primary neoplastic cervical mass and its pulmonary metastases consistent with MTC in a young dog.
A 3-year-old, 21 kg, spayed female, mixed breed dog presented to the Veterinary Teaching Hospital at Virginia-Maryland Regional College of Veterinary Medicine for a slow growing mass that had been present for three months. Upon initial examination, radiography revealed a 5.5 × 5.4 × 4.0 cm, poorly defined, round, and irregularly marginated mass that extended from the left ventrolateral aspect of the larynx and involved the dorsal aspect of the left thyroid bone of the hyoid apparatus (Fig. 1A). The primary mass contained multiple small areas of mineralization (Fig. 1B), and there was no evidence of pulmonary metastasis. Based on clinical and radiological examinations, the mass was deemed non-resectable; however, surgical removal was attempted due to slight compression of the larynx and compromised swallowing. The mass was highly vascularized and infiltrated the adjacent laryngeal cartilage and local cervical musculature. Although there were no signs of metastases and the patient showed mild to no clinical signs related to the primary mass, the owners elected euthanasia due to a poor prognosis associated with invasion and inability to completely resect the mass.
Postmortem examination revealed a hard, immobile, 7.0 cm diameter mass caudal to the ramus of the mandibles spanning the width of the trachea. The mass compromised the left hyoid apparatus and compressed the left peri-laryngeal region, resulting in partial collapse of the laryngeal inlet (A and B in Fig. 2). A cross section revealed that the mass had a large friable necrotic center intermixed with areas of ossification (Fig. 2B). The submandibular and retropharyngeal lymph nodes were bilaterally firm and enlarged (3.0 cm diameter). Additionally, there were numerous off-white, smooth, firm masses varying from a few millimeters to 1.0 cm in diameter scattered throughout all lung lobes (Fig. 2C).
Histologically, the cervical mass represented a densely cellular, nonencapsulated neoplasm composed of cells arranged in large sheets separated by abundant fibrous connective tissue (Fig. 3A). The cells exhibited marked pleomorphism varying from plump elongated to polygonal, had indistinct borders, and a round to ovoid nucleus containing one to a few small variably sized nucleoli. Rarely, the cells tended to pallisade around small segments of fibrovascular stroma (Fig. 3B). Anisokaryosis was moderate and there were 19 mitotic figures in ten 400x fields. Large areas of necrosis disrupted the neoplasm, and there were small islands of partially mineralized osteoids that occasionally entrapped clusters of the neoplastic cells or contained small, normal, osteocytes (Fig. 3B). The lungs were multifocally disrupted by well-delineated, unencapsulated, cell-dense masses composed of cells arranged in packets or nests supported by a thin fibrovascular stroma (C and D in Fig. 3). Neoplastic cells were polyhedral, had indistinct borders, and contained a round, dense, basophilic and central nucleus. There was one mitotic figure in ten 400× fields (Fig. 3D). These histological features were most consistent with a primary cervical neuroendocrine carcinoma with osseous metaplasia and pulmonary metastasis. The presence of amyloid was evaluated under polarized light in sections, with and without 5% potassium permanganate pretreatment, and stained with Congo red.
The stroma of the primary laryngeal mass contains large areas of apple green birefringence congophilic material; however, no areas of polarization were observed in sections of the metastatic pulmonary masses. In sections of the laryngeal mass and lung, neoplastic cells were positive for vimentin, chromogranin A, neuron-specific enolase (NSE), and calcitonin (Figs. 4 and 5), while they were negative for cytokeratins, synaptophysin, thyroglobulin, CD79a, and CD3. Based on this immunohistochemical profile (Table 1), the cervical neuroendocrine carcinoma was further classified as a medullary thyroid carcinoma with pulmonary metastases.
MTC has been widely described in aged bulls, cows, horses, rats, ferrets, red foxes and mouflons [2,6,8,10,12], but only few reports describing these tumors in dogs have been published [3,6,15,16]. The WHO classification scheme describes MTC with variable histological patterns ranging from follicular to solid masses; nevertheless, most reported cases are of the solid type [16]. Similarly, tumors arising from follicular epithelial cells can have varying degrees of differentiation [10]. Diagnosis of MTC is challenging, and the neoplasms may often be misdiagnosed as a solid-type thyroid carcinoma due to their histologic similarity. However, MTC is now more commonly diagnosed owing to the implementation of immunohistochemical stains [3,17,18]. In the current case, although the primary and metastatic masses showed marked pleomorphism and different morphological features, they both had a solid pattern. Histological features in the primary cervical mass (large areas of necrosis, osseous metaplasia, thick fibrous connective tissue, and marked cellular pleomorphism) may have obscured a more typical neuroendocrine pattern.
MTC may be diagnosed on sections stained with H&E; however, immunohistochemistry is often necessary to differentiate it from other neuroendocrine solid patterntype tumors, particularly in metastatic lesions [16]. Although the presence of amyloid deposits in the stroma is a diagnostic feature of MTC in humans, the presence of this feature is inconsistent within the veterinary literature. In this case, the presence of red congophilic material was clear in the primary mass, but there was no evidence of amyloid material in the metastatic masses. Immunoreactivity with calcitonin is the most reliable feature for diagnosis of MTC; however, additional staining with other markers, including chromogranin, NSE, synaptophysin, calcitonin generelated peptide, and carcinoembryonic antigen can often provide a more accurate diagnosis [4,5]. Although calcitonin immunoreactivity is highly specific for MTC, the staining pattern may vary from a diffuse pattern throughout the mass to focal staining with ≤25% of neoplastic cells exhibiting cytoplasmic reactivity [11]. In the current case, 100% of the neoplastic cells showed strong immunoreactivity. Dogs with MTC usually have high levels of circulating calcitonin, but normal levels of calcium and phosphorus. In a few cases, hypocalcaemia and normophosphatemia were reported in conjunction with hypercalcitoninemia, which was resolved after surgical removal of the primary tumor [18]. The biochemical profile in the current case was unremarkable. While noncalcitonin-producing MTCs have been described in human medicine [19], non-productive MTCs have not been reported in the veterinary literature. Chromogranin may be more sensitive, but less specific than calcitonin for the diagnosis of MTC [4]. Although NSE is mostly found in neurons, it is also present within neuroendocrine cells; therefore, it is often used as a marker of neuroendocrine origin, regardless of the specific hormone stored in the cells. Owing to the different degrees of cellular differentiation observed in MTC, the intensity and percentage of positive cells for NSE might vary from 20~100% [11]. In this case, marked cellular pleomorphism was observed within the primary tumor and there were morphological cellular differences within the metastatic masses, but they both showed some degree of functional differentiation since 100% of the neoplastic cells exhibited strong immunoreactivity against NSE. Synaptophysin is a commonly expressed glycoprotein in neurons that can also serve as a good marker for MTC when employed along with calcitonin [17].
It is well known that thyroglobulin antibodies are highly specific for follicular cells of the thyroid gland. C-cells in MTC can proliferate and overcome thyroid follicular cells, resulting in their becoming barely detectable, with reported positive immunoreactivity rates of only up to 10% [4,16]. In this case, the lack of histologically detectable follicular structures and thyroglobulin-positive thyroid cells further confirms their C-cell origin. MTCs normally metastasize to local lymph nodes and lungs, while few reports have described metastasis to the prostate and spleen [7].
Gross, histological, and immunohistochemical findings in this case were consistent with MTC, although there were a few unique features of the tumor in this case. Local infiltration of the primary cervical mass was so severe that surgical resection was not possible, and metastases to local lymph nodes and lungs were consistent with other previously described cases [10]. While the cells of the primary and metastatic masses differed in their histological appearance, they both showed the same immunohistochemical profile, further confirming MTC. While neoplasms of thyroid gland have distinctive features that allows its histological differentiation, the final diagnosis and classification of thyroid gland neoplasms is still being challenging. Therefore, further investigation with multiple immunohistochemical markers is necessary to differentiate and classify thyroid neoplasms.
Figures and Tables
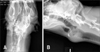 | Fig. 1Radiographic images of a large tissue-dense cervical mass on the laryngeal region. The dorso-ventral image (A) showed a faint circular irregular opacity that extends from C1 to the inter-mandibular region. In the latero-lateral image (B), the osseous mass overlaps the larynx and extends caudal to the second tracheal ring. |
 | Fig. 2(A) Neoplastic mass protruding from the left peri- laryngeal region compressing the laryngeal inlet. (B) The mass was ossified, firmly adhered to the larynx and entrapped the left hyoid bone. There were large necrotic centers on the cut section. (C) Multiple, variable sized, firm nodules were scattered throughout the lungs. |
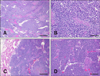 | Fig. 3(A) The cervical mass was composed of large sheets of densely cellular masses supported by abundant fibrous tissues that were disrupted by areas of necrosis and numerous small osseous spicules and areas or mineralization. (B) Cells were pleomorphic, varying form polyhedral to plump elongated, and pallisaded around a small area of the fibrovascular stroma. (C) Sections of lung showed well-demarcated non-encapsulated, densely cellular masses. (D) Neoplastic masses were composed of nests and islands of polyhedral cells supported by thin vascular stroma. Scale bars = 200 µm (A and C), 100 µm (B and D). |
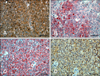 | Fig. 4Immunohistochemical panel showing the intensity and percentage of positive cells in the primary mass for (A) chromogranin A, (B) neuron-specific enolase (NSE), (C) vimentin and (D) calcitonin. Scale bars = 50 µm. |
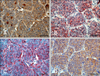 | Fig. 5Immunohistochemical panel showing the intensity and percentage of positive cells in the pulmonary metastatic nodules for (A) chromogranin A, (B) neuron-specific enolase (NSE), (C) vimentin and (D) calcitonin. Scale bars = 50 µm. |
Acknowledgments
We thank Dr. Carley Allen (Virginia-Maryland Regional College of Veterinary Medicine, Virginia Tech) for the clinical presentation of this case. Dr. Monica Diessler (Catedra de Histologia, Facultad de Ciencias Veterinarias, Universidad Nacional de la Plata) and Dr. Phillip Sponenberg (Department of Biomedical Sciences and Pathobiology, VA-MD Regional College of Veterinary Medicine) conducted critical revisions of this manuscript. We thank Barbara J. Wheeler, Jennifer Rudd (VA-MD Regional College of Veterinary Medicine), and Dee DuSold (Animal Disease Diagnostic Laboratory, Purdue University) for their technical assistance with immunohistochemical procedures.
References
1. Barber LG. Thyroid tumors in dogs and cats. Vet Clin North Am Small Anim Pract. 2007; 37:755–773.

2. Boorman GA, Hollander CF. Animal model of human disease: medullary carcinoma of the thyroid. Am J Pathol. 1976; 83:237–240.
3. Carver JR, Kapatkin A, Patnaik AK. A comparison of medullary thyroid carcinoma and thyroid adenocarcinoma in dogs: a retrospective study of 38 cases. Vet Surg. 1995; 24:315–319.

4. Erickson LA, Lloyd RV. Practical markers used in the diagnosis of endocrine tumors. Adv Anat Pathol. 2004; 11:175–189.

5. Fischer S, Asa SL. Application of immunohistochemistry to thyroid neoplasms. Arch Pathol Lab Med. 2008; 132:359–372.

6. Fox JG, Dangler CA, Snyder SB, Richard MJ, Thilsted JP. C-cell carcinoma (medullary thyroid carcinoma) associated with multiple endocrine neoplasms in a ferret (Mustela putorius). Vet Pathol. 2000; 37:278–282.

7. Harmelin A, Nyska A, Aroch I, Yakobson B, Stem S, Orgad U, Waner T. Canine medullary thyroid carcinoma with unusual distant metastases. J Vet Diagn Invest. 1993; 5:284–288.

8. Hirayama K, Kagawa Y, Nihtani K, Taniyama H. Thyroid C-cell carcinoma with amyloid in a red fox (Vulpes vulpes schrenchki). Vet Pathol. 1999; 36:342–344.

9. Holscher MA, Davis BW, Wilson RB, Hunt KL, Berry KK. Ectopic thyroid tumor in a dog: thyroglobulin, calcitonin, and neuron-specific enolase immunocytochemical studies. Vet Pathol. 1986; 23:778–779.

10. Kiupel M, Capen C, Miller M, Smedley R. Histological Classification of Tumors of the Endocrine System of Domestic Animals. Washington DC: Armed Forces Institute of Pathology;2008. Vol. 12:p. 64–84.
11. Krisch K, Krisch I, Horvat G, Neuhold N, Ulrich W. The value of immunohistochemistry in medullary thyroid carcinoma: a systematic study of 30 cases. Histopathology. 1985; 9:1077–1089.

12. Kuwamura M, Shirota A, Yamate J, Kotani T, Ohashi F, Sakuma S. C-cell adenoma containing variously sized thyroid follicles in a horse. J Vet Med Sci. 1998; 60:387–389.

13. Leblanc B, Parodi AL, Lagadic M, Hurtrel M, Jobit C. Immunocytochemistry of canine thyroid tumors. Vet Pathol. 1991; 28:370–380.

14. Leblanc B, Paulus G, Andreu M, Bonnet MC. Immunocytochemistry of thyroid C-cell complexes in dogs. Vet Pathol. 1990; 27:445–452.

15. Lee JJ, Larsson C, Lui WO, Höög A, Von Euler H. A dog pedigree with familial medullary thyroid cancer. Int J Oncol. 2006; 29:1173–1182.

16. Moore FM, Kledzik GS, Wolfe HJ, DeLellis RA. Thyroglobulin and calcitonin immunoreactivity in canine thyroid carcinomas. Vet Pathol. 1984; 21:168–173.

17. Patnaik AK, Lieberman PH. Gross, histologic, cytochemical, and immunocytochemical study of medullary thyroid carcinoma in sixteen dogs. Vet Pathol. 1991; 28:223–233.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download