Abstract
The objectives of the present study were to evaluate the anatomic localization of porcine reproductive and respiratory syndrome virus (PRRSV) in naturally infected pigs and to determine whether oral fluid could be used to detect the virus in infected animals. Two sows, seven 2-month-old grower pigs, and 70 6-month-old gilts were included in this study. PRRSV in sera and oral fluid were identified by nested reverse transcription PCR (nRT-PCR) while lung, tonsil, and tissue associated with oral cavity were subjected to nRT-PCR, immunohistochemistry, and in situ hybridization. In sows, PRRSV was identified in oral fluid and tonsils. PRRSV was also detected in oral fluid, tonsils, salivary glands, oral mucosa, and lungs of all seven grower pigs. However, viremia was observed in only two grower pigs. Double staining revealed that PRRSV was distributed in macrophages within and adjacent to the tonsillar crypt epithelium. In gilts, the North American type PRRSV field strain was detected 3 to 8 weeks after introducing these animals onto the farm. These results confirm previous findings that PRRSV primarily replicates in tonsils and is then shed into oral fluid. Therefore, oral fluid sampling may be effective for the surveillance of PRRSV in breeding herds.
Porcine reproductive and respiratory syndrome virus (PRRSV) was first reported in North America in 1987, and is one of the most economically important diseases in the swine industry [16]. Infection with PRRSV is characterized by two clinical signs: reproductive failure in sows during the early-to-late stage of gestation and respiratory problems in piglets at any age [7,11,16,17]. Numerous studies have been conducted to determine the localization of the virus in experimental pigs. In boars, PRRSV appears to persist in lymphoid tissues, particularly in tonsils, rather than the reproductive system [9]. A study of stillborn and live birth piglets from intranasally inoculated sows indicated that PRRSV replicates primarily in lymphoid tissues before the virus affects internal organs such as the lungs, heart, liver, spleen, and kidneys [7]. In experimentally infected sows, PRRSV was detected in the tonsils, heart, uterus, kidneys, and lymph nodes [3]. However, little information is available on localization of PRRSV in sows in the field.
PRRSV was first reported in oral fluid in 1997 [21]. Recently, oral fluid sampling has been used to examine swine populations [12,14,15]. There are several situations in which oral fluid sampling may be advantageous for a breeding herd since this method can be performed more frequently at any time during gestation without jeopardizing the welfare of the animals. Although it has been reported that oral fluid has the potential for replacing serum to detect PRRSV in experimental pigs [12,15], the source of PRRSV in oral fluid has not been determined.
In breeding herds, gilt introduction is a very important factor for PRRSV control. Gilts are susceptible to PRRSV infection and are a potential reservoir of the virus in the herd if they become viremic during the breeding via horizontal as well as vertical viral transmission. The goal of a PRRSV acclimatization program is to expose gilts to the same strain of virus to which the herd is resistant. This allows sufficient time to permit full recovery of the gilts before their introduction into the breeding herd. Information on natural PRRSV infection in replacement gilts during the acclimatization period would be useful for establishing a suitable acclimatization program and PRRSV elimination strategy. Therefore, the goals of the present study were to clarify the localization of PRRSV in infected swine and to evaluate the use of oral fluid specimens for PRRSV detection in the field.
Pigs were collected from a 400-sow herd isolated from other pig farms in Miyazaki, Japan. Reduction in the farrowing rate and increased incidences of abortion were observed among the sows. The herd was considered to be endemically infected with PRRSV based on diagnostic history and on-going surveillance.
Two 3-year-old pregnant sows (A and B) that naturally aborted after 76 and 92 days of gestation along with seven 2-month-old grower pigs with respiratory problems were euthanized with mafropane (DS Pharma Animal Health, Japan) and subjected to necropsy. One hour prior to euthanasia, serum and oral fluid were collected. Samples of lungs, brain, heart, liver, spleen, kidneys, ileum, and tissues from the oral cavity (i.e., tonsils, salivary glands, submandibular lymph nodes, and oral mucosa) were taken from each animal. Additional samples of the uterus and ovaries were collected from the sows. Eight fetuses were randomly selected from the sow litters to survey for PRRSV with nested reverse transcription PCR (nRT-PCR).
In order to assess PRRSV infection during the acclimatization period, 70 6-month-old PRRSV-free gilts were purchased from Zen-noh livestock (Japan) and housed in seven pens (10 gilts/pen) in an isolated acclimation building at the farthest corner of the farm. Disinfectant footbaths and changing coveralls (Kenis, Japan) were used prior to entering each pen. During the 3-week period after arrival, the technician worked in other pig buildings before going to the acclimation zone without changing coveralls in order to introduce gilts to the current PRRSV strain circulating in the farm.
The gilts were vaccinated with a PRRSV modified live vaccine 2 mL dose (Ingelvac PRRS MLV; Boehringer Ingelheim Vetmedica, USA) three times 1 day after arrival, 4 weeks after the first injection, and 3 weeks before first round of insemination. In the breeding herd, sows were vaccinated every 3 months as recommended by the manufacturer. The vaccine was not administered to piglets, grower animals, or finisher pigs. Oral fluids were collected using a sterile cotton rope (100% cotton rope; Kenis) to absorb the fluid in the oral cavity [12,15] from pigs in each pen at 1 week intervals for 11 weeks. Serum samples were collected from individual gilts by using serum separator tube (Venoject II; Terumo Medical Corporation, USA) on the first and eighth weeks of the acclimatization period. PRRSV antibody in serum was identified by an enzyme-linked immunosorbent assay (ELISA) kit (AniGen PRRS Ab ELISA kit; Bionote, Korean) according to the manufacturer's instructions.
The expression of PRRSV RNA was examined by nRT-PCR. RNA from the tissue samples was extracted with TRIzol (TRIzol reagent; Invitrogen, USA) while that in sera and oral fluid was extracted with viral RNA extraction kit (QIAamp Viral RNA Mini Kit; Qiagen, Germany) according to the manufacturer's instructions. Two PCR primer pairs were used for nRT-PCR. The first PCR primers were 13586F (5'-GTGGTATTTGGCAATG TGTC-3')/14652R (5'-CTCCAGGTTTCTATGGCTGA-3'), which amplified 1067 bp of the open reading frame (ORF) 4-6 region of PRRSV [22]. The second primers, P420F (5'-CCATTCTGTTGGCAATTTGA-3')/P620R (5'-GGCATATATCATCACTGGCG-3'), which amplified 713 bp of the first RT-PCR product [1]. The first RT-PCR was performed with AccessQuick RT-PCR System (Promega, USA) and the second PCR was performed with GoTaq Green Master Mix (Promega). The first RT-PCR reaction was carried out for one cycle of reverse transcription at 45℃ for 45 min, inactivation at 94℃ in 2 min, and 40 cycles of denaturation at 94℃ for 15 sec, annealing at 55℃ for 30 sec, extension at 68℃ for 1 min, and final incubation at 72℃ for 5 min. The second PCR reaction was performed as first denaturation at 94℃ for 2 min, on 40 cycles of denaturation at 94℃ for 15 sec, annealing at 55℃ for 30 sec, extension at 68℃ for 1 min, and following the final incubation at 72℃ for 5 min. Multiplex PCR and multiplex RT-PCR were also performed as previously described [13] to screen for other pathogens including porcine circovirus type 2 (PCV2), suid herpesvirus 1, porcine parvovirus, Getah virus, and Japanese encephalitis virus.
The North American (NA)-type PRRSV open reading frame (ORF) 5 gene was sequenced with AbI Prism BigDye Terminator v3.1 Cycle Sequencing Kits (Applied Biosystems, USA) using primer P420F/P620R (Sigma-Aldrich, USA) [1]. Sequence of the vaccine strain (Accession No. AF066183, University of Minnesota, USA) was obtained from GenBank. Sequence data were analyzed with GENETYX (ver. 7.0; Genetyx Corporation, Japan). Alignment was performed with isolates in this study, 3 previous isolates from the farm (Farm10/2011, Farm 11/2011, and Farm 12/2011), and the inoculated vaccine strain. A phylogenetic tree was constructed using the neighbor-joining method and a bootstrap analysis was carried out with 1,000 replications.
Tissue samples from the sows and grower pigs were fixed in 4% paraformaldehyde for one day then embedded in paraffin by standard histologic procedures. Two-micrometer-thick tissue sections were made for hematoxylin and eosin (HE), immunohistochemistry (IHC), in situ hybridization (ISH), double IHC-ISH, and double immunofluorescence stain. For IHC, antigens were retrieved by incubation with 20 µg/mL proteinase K (Wako, Japan) at 37℃ in 10 min. Endogenous peroxidase activity was suppressed by 3% hydrogen peroxide in methanol for 30 min at room temperature. Non-specific binding was inhibited with a blocking reagent (Blocking one; Nacalai Tesque, Japan) for 30 min at 37℃ in moist chamber. The tissue samples were then incubated at 4℃ overnight with a monoclonal anti-PRRSV antibody SR30 (dilute 1 : 10,000 in phosphate-buffered saline [PBS]; Rural Technologies, USA). Next, the sections were incubated with EnVision (DakoCytomation, Japan) as a secondary antibody for 30 min at 37℃. After each incubation period, the sections were washed three times with PBS. Finally, the sections were stained using a peroxidase stain diaminobenzidine (DAB) kit (Nacalai Tesque).
For ISH, antisense cRNA probes specific for PRRSV RNA were synthesized from the ORF 7 gene sequence as previously described [19]. Digoxigenin (DIG)-labeled cRNA probes were prepared using a DIG RNA labeling kit (Roche Diagnostics, Germany). ISH specific for PRRSV was performed as previously described by Tanizaki et al. [19] Tissues positive for PRRSV according to IHC and/or ISH were further subjected to double staining to confirm whether macrophages were the target cells for PRRSV.
For double IHC-ISH, the tissue sections were first processed for IHC staining using lysozyme EC 3.2.1.17 (dilute 1 : 1,000 in PBS; Dako, Denmark) as a macrophage marker. Sections were incubated with an universal immnuno-alkaline-phosphatase polymer, anti-mouse and -rabbit (Histofine simple stain AP (MULTI); Nichirei, Japan) for 30 min at 37℃. Immnunoreactivity was visualized by Fast Red II (Nichirei). Consequently, sections were process for ISH using PRRSV RNA probe. Finally, color was developed using the DAB substrate (Liquid DAB+Substrate Chromogen System; DakoCytomation) and counterstained with hematoxylin.
Double immunofluorescence labeling was performed with lysozyme EC 3.2.1.17 (dilute 1 : 1,000 in PBS) and fluorescein isothiocyanate (FITC)-conjugated polyclonal swine anti-rabbit IgG (dilute 1 : 40 in PBS; DakoCytomation). The sections were subsequently incubated with the monoclonal anti-PRRSV antibody SR30 (dilute 1 : 10,000) followed by Alexa Fluor 594 (dilute 1 : 200, Molecular Probes, USA) at room temperature in dark moist chamber for 60 min each antibody. Finally, the tissue sections were mounted using Vectashield with 4',6-diamidino-2-phenylindole (Vector Laboratories, USA). Confocal microscope (A1+ confocal microscope; Nikon, Japan) was used to view and analyze the stained cells.
Sows that had aborted did so without showing other clinical signs of disease. PRRSV RNA was identified in the oral fluid and tonsils of both sows but not in the sera, salivary glands, uterus, or other organs (Table 1). Viral RNA was detected in lungs and placentas of three out of eight fetuses from these sows. Histologically, tonsils from the sows were characterized by mild infiltration of macrophages, neutrophils, lymphocytes, and cryptal epithelial cells in the dilated tonsillar crypts. No lesions were observe in the lungs or uterus of these sows. With ISH, PRRSV RNA was detected in the cytoplasm of large oval cells that possessed morphological features similar to those of macrophages or dendritic cells near tonsillar crypts. PRRSV antigens, however, were not identified by IHC in formalin-fixed tissues from the sows.
In all seven grower pigs, lesions were mainly observed in cranioventral lung lobes that were characterized by consolidation, discoloration, and failure to collapse when the thorax was opened. Additionally, rib imprints were found in two pigs. Microscopically, all seven animals were found to have interstitial pneumonia based on evaluation of the lung samples. Alveolar walls were thickened due to the infiltration of macrophages, lymphocytes, and neutrophils. Tonsillitis in the grower pigs was characterized by erosion of the crypt epithelia and dilated crypt lumina filled with macrophages, neutrophils, lymphocytes, and cryptal epithelial cells (A and B in Fig. 1).
A few PRRSV-positive cells were found in the tonsillar cryptal lumen by ISH (C and D in Fig. 1). PRRSV was consistently detected in the tonsils, oral fluid, salivary glands, and lungs of all seven grower pigs along with the oral mucosae and submandibular lymph nodes of six grower pigs. In contrast, only two serum samples were positive for the virus as detected by nRT-PCR. Viral antigen and nucleic acids were detected in the lungs (grower pigs 1 and 6) and tonsils (grower pigs 1 and 3) by both IHC and ISH (Table 1). None of the samples from the brain, heart, liver, spleen, kidney, or ileum obtained from these grower pigs was positive for PRRSV. No other viruses, including PCV2, suid herpesvirus 1, porcine parvovirus, Getah virus, and Japanese encephalitis virus, were detected in any samples from the sows or grower pigs.
Sections of tonsils from the sows and grower pigs were double stained to identify target cells of PRRSV. In the double labeling IHC-ISH experiment, macrophages and PRRSV RNA were stained red or brown, respectively. Double IHC-ISH findings revealed that PRRSV RNA was distributed in the cytoplasm of macrophages within and adjacent to the crypt epithelium (Fig. 2). Double immunofluorescence produced positive signals for PRRSV antigen and lysozymes that appeared as red or green, respectively. PRRSV antigen that colocalized with lysozymes was visualized as red and green or orange signals in the cytoplasm of macrophages near the tonsillar crypts (Fig. 3).
The course of PRRSV infection in replacement gilts during acclimatization is shown in Table 2. At the first week following introduction, oral fluid was collected from animals in three out of seven pens. After the first collection, the pigs became more familiar with the rope and oral fluid samples from all animals could be collected. PRRSV RNA was detected in the serum of one gilt 1 week after the live vaccine was injected. Three weeks post-introduction, the gilts were anorexic and PRRSV was detected in oral fluid from the animals in three pens. By week 4, gilts in all seven pens were infected by the virus. The periods during which the virus could be detected in oral fluid differed from pen to pen and ranged from 3 to 6 weeks. Virus was detected in oral fluid from the pigs in five pens until week 8, but was not found in any serum samples taken from individual gilts. PRRSV was not identified in oral fluid samples obtained between week 9 and week 11. At week 1, all gilts were seronegative with sample : positive (S : P) ratios ≤ 0.4. By week 8, the anti-PRRSV antibody levels had increased with mean S : P ratios ≥ 2.6 indicating that all gilts had seroconverted.
PRRSV isolates from tonsils of the sows and grower pigs or oral fluids from the gilts were sequenced. Percent identity of ORF 5 nucleotide sequences among virus from the sows, grower pigs, gilts, and previous isolates from the farm (Farm10/2011, Farm 11/2011, and Farm 12/2011) ranged from 95% to 100%. In contrast, all sequence from this farm shared an 87% to 89% nucleotide identity with the inoculated vaccine strain. Thus, it was concluded that the replacement gilts were infected with the same virus strain that exist in the farm (Fig. 4).
In order to determine the localization of PRRSV, sera and tissue samples from two sows that had naturally aborted and seven grower pigs were examined. In sows, viral nucleic acids were detected in the absence of gross and microscopic lesions with the exception of mild tonsillitis. Similar to previous studies, abortion associated with PRRSV infection was not associated with the appearance of lesions [2,6,8]. In contrast to other reports [3,4,7]. PRRSV was only detected in the tonsils of sows. An acceptable explanation for these observations may be that the present study was conducted on naturally infected sows whereas previous investigations examined experimentally infected sows.
PRRSV was more widely distributed in grower pigs compare to sows. One possible reason is that the sows were vaccinated four times per year whereas grower pigs were not vaccinated. Therefore, immune responses to PRRSV in the sows might have been stronger than that in grower pigs. Previous studies [7,9] demonstrated that PRRSV primarily replicates in tonsils and then spreads to other organs. Therefore, tonsils may also be an important site for PRRSV replication in naturally infected sows and grower pigs. Double immunofluorescence showed that PRRSV antigen is also present in the cytoplasm of macrophages. It is widely recognized that macrophages are the target cells for PRRSV replication [17].
Traditionally, serum has been the specimen of choice for detecting various pathogens including PRRSV. Oral fluids are increasingly being used for analysis in the swine industry since they are easily collected from individual pigs and are suitable for pen-based sampling. In the current study, PRRSV was examined in oral fluid and serum from PRRSV-free gilts during acclimatization. One week following inoculation, the virus was detected in the serum of one gilt. Unfortunately, sequencing could not be performed for this sample to confirm whether the gilt was infected by the vaccine strain or field strain due to a low RNA concentration. Because a modified live PRRSV vaccine was used, it is probable that the gilt was infected by the vaccine strain. One week post-vaccination, the gilt may have been in the acute phase of infection. In agreement with findings from a previous study [18], the virus was detected in the serum of gilts 3 days post-vaccination. During acute infection, serum was found to be superior to oral fluid for early detection of PRRSV [14]. However, PRRSV was identified week 8 post-arrival in five out of seven oral fluid samples from the gilts but none of the serum samples from all 70 swine. PRRSV was detected in all oral fluid samples from the grower pigs but in only two out of seven serum samples. For the sows, PRRSV RNA was detectable in both oral fluid specimens but was not found in serum. Therefore, analysis of oral fluid specimens could be used as an alternative to serum sampling to identify PRRSV in cases of persistent infection.
Early in the acclimatization period, gilts can be exposed to viruses through vaccination or live virus injection [10] as well as exposure to viremic nursery pigs [20]. Live virus injection is associated with potential risks such as the spread of other pathogens and increased mortality. In the current study, gilts were introduced to a farm where PRRSV was endemic and a modified live vaccine was used to exposure the gilts to the virus. However, the gilts were infected by the PRRSV field strain 3 weeks post-introduction. Thus, the gilts may have been infected before the vaccine-induced immune response to the virus was initiated. Moreover, nucleotide sequencing data indicated that the gilts were infected by the field strain, which shared only a 87% to 89% nucleotide identity with the vaccine strain. A previous investigation [18] demonstrated that the degree of protection conferred by vaccination depends on the degree of similarity between the PRRSV vaccine strain and field strain to which the pigs are exposed. Therefore, the current vaccine that contains a single strain of PRRSV might not be effective for protecting the animals against infection with genetically different strains of PRRSV.
The gilts were housed in an isolated acclimatization building. The source of infection in this study may have involved indirect routes of transmission such as ones associated with contaminated fomites. Indirect transmission might be a useful method for exposing replacement gilts to the current PRRSV circulating in the farm and inducing an immune response before entering the PRRSV-positive breeding herd. This technique may also reduce the risk of transmission of other pathogens unlike exposure to viremic pigs or live virus injection. Further studies are necessary to confirm the effect of indirect transmission on replacement gilt immunization.
In conclusion, our finding indicated that PRRSV primarily replicates in tonsils. The virus is then shed into oral fluid. Therefore, oral fluid sampling could be suitable for PRRSV surveillance in breeding herds.
Figures and Tables
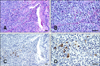 | Fig. 1Tonsil tissue from piglet 1 naturally infected with porcine reproductive and respiratory syndrome virus (PRRSV). Tonsillar crypt with macrophages, neutrophils, lymphocytes, and cryptal epithelial cells in the lumen. (A) Hematoxylin and eosin staining. (B) Higher magnification. PRRSV nucleic acids were detected in the cytoplasm of cells resembling macrophages in the tonsillar crypts. (C) ISH and hematoxylin counterstaining. (D) Higher magnification. Scale bars = 10 µm. |
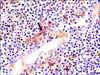 | Fig. 2Tonsil section from piglet 1 naturally infected with PRRSV. PRRSV nucleic acids were stained brown and lysozymes were stained red. Double-positive cells showed mixed red and brown staining in the cytoplasm (arrow). Double labeling of the lysozymes with IHC and PRRSV RNA using ISH. Hematoxylin counterstaining. Scale bars = 10 µm. |
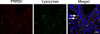 | Fig. 3Tonsil section from piglet 7 naturally infected with PRRSV. Double immunofluorescence staining for PRRSV antigen (red) and lysozymes (green). Double-positive cells contained both red and green signals or orange fluorescence in the cytoplasm (arrows). Nuclei were counterstained with 4',6-diamidino-2-phenylindole (blue). Scale bars = 10 µm. |
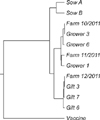 | Fig. 4Phylogenetic tree showing the relationships based on the North American-type PRRSV ORF 5 nucleotide sequences. The tree was constructed using the neighbor-joining method for eight isolates recovered in this study (Sow A, Sow B, Gilt 3, Gilt 6, Gilt 7, Grower 1, Grower 3, and Grower 6), the vaccine strain (Vaccine), and three previously obtained PRRSV isolates from the farm (Farm 10/2011, Farm 11/2011, and Farm 12/2011). A bootstrap analysis was performed with 1,000 replicates. |
Acknowledgments
This study was supported by a Japanese Society for the Promotion of Science (JSPS, Grant No. 23580425).
References
1. Andreyev VG, Wesley RD, Mengeling WL, Vorwald AC, Lager KM. Genetic variation and phylogenetic relationships of 22 porcine reproductive and respiratory syndrome virus (PRRSV) field strains based on sequence analysis of open reading frame 5. Arch Virol. 1997; 142:993–1001.

2. Benson JE, Yaeger MJ, Christopher-Hennings J, Lager K, Yoon KJ. A comparison of virus isolation, immunohistochemistry, fetal serology, and reverse-transcription polymerase chain reaction assay for the identification of porcine reproductive and respiratory syndrome virus transplacental infection in the fetus. J Vet Diagn Invest. 2002; 14:8–14.

3. Bierk MD, Dee SA, Rossow KD, Collins JE, Guedes MI, Molitor TW. Experiences with tonsil biopsy as an antemortem diagnostic test for detecting porcine reproductive and respiratory syndrome virus infection in breeding swine. Swine Health Prod. 2000; 8:279–282.
4. Bierk MD, Dee SA, Rossow KD, Collins JE, Guedes MI, Pijoan C, Molitor TW. Diagnostic investigation of chronic porcine reproductive and respiratory syndrome virus in a breeding herd of pigs. Vet Rec. 2001; 148:687–690.

5. Choi C, Chae C. Colocalization of porcine reproductive and respiratory syndrome virus and porcine circovirus 2 in porcine dermatitis and nephrology syndrome by double-labeling technique. Vet Pathol. 2001; 38:436–441.

6. Cheon DS, Chae C. Comparison of virus isolation, reverse transcription-polymerase chain reaction, immunohistochemistry, and in situ hybridization for the detection of porcine reproductive and respiratory syndrome virus from naturally aborted fetuses and stillborn piglets. J Vet Diagn Invest. 2000; 12:582–587.

7. Cheon DS, Chae C. Distribution of porcine reproductive and respiratory syndrome virus in stillborn and liveborn piglets from experimentally infected sows. J Comp Pathol. 2001; 124:231–237.

8. Christianson WT, Collins JE, Benfield DA, Harris L, Gorcyca DE, Chladek DW, Morrison RB, Joo HS. Experimental reproduction of swine infertility and respiratory syndrome in pregnant sows. Am J Vet Res. 1992; 53:485–488.
9. Christopher-Hennings J, Holler LD, Benfield DA, Nelson EA. Detection and duration of porcine reproductive and respiratory syndrome virus in semen, serum, peripheral blood mononuclear cells, and tissues from Yorkshire, Hampshire, and Landrace boars. J Vet Diagn Invest. 2001; 13:133–142.

10. Fano E, Olea L, Pijoan C. Eradication of porcine reproductive and respiratory syndrome virus by serum inoculation of naïve gilts. Can J Vet Res. 2005; 69:71–74.
11. Karniychuk UU, Saha D, Geldhof M, Vanhee M, Cornillie P, Van den Broeck W, Nauwynck HJ. Porcine reproductive and respiratory syndrome virus (PRRSV) causes apoptosis during its replication in fetal implantation sites. Microb Pathog. 2011; 51:194–202.

12. Kittawornrat A, Prickett J, Chittick W, Wang C, Engle M, Johnson J, Patnayak D, Schwartz T, Whitney D, Olsen C, Schwartz K, Zimmerman J. Porcine reproductive and respiratory syndrome virus (PRRSV) in serum and oral fluid samples from individual boars: will oral fluid replace serum for PRRSV surveillance? Virus Res. 2010; 154:170–176.

13. Ogawa H, Taira O, Hirai T, Takeuchi H, Nagao A, Ishikawa Y, Tuchiya K, Nunoya T, Ueda S. Multiplex PCR and multiplex RT-PCR for inclusive detection of major swine DNA and RNA viruses in pigs with multiple infections. J Virol Methods. 2009; 160:210–214.

14. Pepin BJ, Kittawornrat A, Liu F, Gauger PC, Harmon K, Abate S, Main R, Garton C, Hargrove J, Rademacher C, Ramirez A, Zimmerman J. Comparison of specimens for detection of Porcine reproductive and respiratory syndrome virus infection in boar studs. Transbound Emerg Dis. 2013; Epub ahead of print. doi:10.1111/tbed.12135.

15. Prickett JR, Simer R, Christopher-Hennings J, Yoon KJ, Evans RB, Zimmerman JJ. Detection of Porcine reproductive and respiratory syndrome virus infection in porcine oral fluid samples: a longitudinal study under experimental conditions. J Vet Diagn Invest. 2008; 20:156–163.

17. Rowland RRR, Lawson S, Rossow K, Benfield DA. Lymphoid tissue tropism of porcine reproductive and respiratory syndrome virus replication during persistent infection of pigs originally exposed to virus in utero. Vet Microbiol. 2003; 96:219–235.

18. Scortti M, Prieto C, Simarro I, Castro JM. Reproductive performance of gilts following vaccination and subsequent heterologous challenge with European strains of porcine reproductive and respiratory syndrome virus. Theriogenology. 2006; 66:1884–1893.

19. Tanizaki Y, Sato Y, Oka H, Utsuki S, Kondo K, Miyajima Y, Nagashio R, Fujii K. Expression of autocrine motility factor mRNA is a poor prognostic factor in high-grade astrocytoma. Pathol Int. 2006; 56:510–515.

20. Vashisht K, Erlandson KR, Firkins LD, Zuckermann FA, Goldberg TL. Evaluation of contact exposure as a method for acclimatizing growing pigs to porcine reproductive and respiratory syndrome virus. J Am Vet Med Assoc. 2008; 232:1530–1535.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download