Abstract
Here we report the detection and distribution of synaptophysin (SPY), non-neuronal enolase (NNE), glial fibrillary acidic protein (GFAP), vimentin (VIM), neuropeptide Y (NPY), and vasoactive intestinal peptide (VIP) expression in the goat forestomach during prenatal development. A total of 140 embryos and fetuses were examined to evaluate protein expression from the first stage of prenatal life until birth. In all cases, SPY immunoreactivity was detected at 53 days gestation in the lamina propria-submucosa, tunica muscularis, serosa, and myenteric plexuses. Immunoreactivity to NNE was observed at 64 days gestation in the same locations as well as the epithelial layer. Glial cells were found at 64 days as indicated by signals corresponding to GFAP and VIM at 39 days. Positive staining for NPY and VIP was observed at 113, 75, and 95 days in the rumen, reticulum, and omasum, respectively, in the lamina propria-submucosa, tunica muscularis, and myenteric plexuses of each of these gastric compartments. These findings indicate possible preparation of the fetal goat forestomach for postnatal function. Compared to other ruminant species, neuroendocrine cells, glial cells and peptidergic innervations markers were detected earlier compared to sheep but at around the same stage as in deer.
The ruminant stomach is particularly remarkable for its ability to transform low-quality forage into products with high nutritional value [20]. This organ is subdivided into four compartments: the rumen, reticulum, omasum, and abomasum. Each compartment is characterized by unique gross and histological features [31] reflecting morphological and functional adaption to the ingestion, processing, and digestion of plant material. Before passing into the abomasum, the final and only truly secretory part of the ruminant stomach, ingesta are subjected to the actions of the other three forestomach compartments [34]. These compartments play a major role in the bacterial digestion of cellulose by acting as fermentation chambers. At the same time, propulsive peristaltic movements, contractions, and the separation of fluids and solids occur [35]. These motor functions require not only coordinated control of the central nervous system but also the presence of an elaborate enteric nervous system [30].
The enteric nervous system is composed of ganglionate plexuses containing neurons and glia situated between the smooth muscle layers of the digestive wall [14]. The functioning of enteric neurons depends on the presence of neurotransmitters [21]. Neuropeptides such as acetylcholine, substance P, neuropeptide Y (NPY), and vasoactive intestinal peptide (VIP) act as putative neurotransmitters in the enteric neurons [21,27]. Glial cells are non-neuronal elements of the enteric nervous system. They are similar to astrocytes of the central nervous system, and most are positive for glial fibrillary acidic protein (GFAP) [36] and vimentin (VIM) [19]. These cells regulate and maintain enteric neuronal activities and support intestinal barrier function [35]. The enteric nervous system also contains endocrine cells capable of secreting hormones. These endocrine cells play a key role in the overall regulation of digestive processes such as nutrient absorption and carbohydrate metabolism homeostasis [4].
In previous immunohistochemical studies, neuropeptides [15,16,17,25,26,27,38], glial cells [36], and endocrine cells [2] were detected in the forestomach of adult ruminants. However, little research examining the prenatal development of these cell types in the stomach of ruminants such as sheep [37], deer [6,7,9,10,28,29], and goats has been conducted [11,12,13]. For the present study, immunohistochemical techniques were used to detect the presence and distribution of the neuroendocrine cell markers synaptophysin (SPY) and non-neuronal enolase (NNE), glial cell markers GFAP and VIM, and peptidergic innervation markers NPY and VIP in the goat forestomach in order to study the nature, origin and evolution of these different cell types during prenatal development.
A total of 140 goat (Capra hircus) embryos and fetuses, representing the earliest prenatal stages to birth, were included in the study. The specimens were divided into five sequential groups according to major histomorphogenic characteristics: group I (crown-rump length [CRL] 1.5~4.3 cm, 13~38 days old, 1~25% gestation), group II (CRL 4.4~8 cm, 39~52 days old, 25~35% gestation), group III (CRL 9~17.5 cm, 53~75 days old, 35~50% gestation), group IV (CRL 18~32 cm, 76~112 days old, 50~75% gestation), and group V (CRL 33~47 cm, 113~150 days old, 75~100% gestation). All embryos and fetuses were obtained from pregnant female goats at a municipal slaughterhouse in Caceres (Spain). The pregnant females were slaughtered according to the usual process of the slaughterhouse. Embryos and fetuses were obtained after opening the abdominal cavity, uterus, and placenta. These procedures were carried out in accordance with guidelines established for the protection of animals at the time of slaughter in slaughterhouses (Spanish Royal Decree 54/1195). Gestational age was estimated according to previously described age classification methods for sheep and goats [5,33]. Six specimens were selected according to three ranges of gestational age with the most revealing histological features within each group for immunohistochemical analysis. In group I, two fetuses 35 days old, two that were 36 days old, and two that were 38 days old were included. Group II consisted of three fetuses 39 days old, two that were 46 days old, and two that were 50 days old. Group III consisted of two fetuses 64 days old, three that were 68 days old, and two that were 70 days old. Group IV included two fetuses 75 days old, two that were 87 days old, and two that were 95 days old. Finally, group V had two fetuses 113 days old, two that were 120 days old, and two that were 150 days old.
The forestomach was extracted and small pieces of tissue (1 square centimeter of surface) from medial region of the dorsal and caudal sac of rumen and medial region of reticulum and omasum were removed for analysis. The tissue samples were washed in phosphate-buffered saline (PBS) and fixed with 4% buffered formaldehyde for 24 h and room temperature. After this time, the tissue samples were subjected to dehydration by successive immersion in ascending concentration of alcohols (70%, 80%, 96%, 100%), xylene and finally, embedded in paraffin. Sections 5-µm thick were cut in transversal direction with microtome (Rotary microtome Leica RM2255; Leica, Germany) from the paraffin block.
An UltraVision One horseradish peroxidase (HRP) polymer system (polymer conjugated to HRP) was used for immunohistochemical analysis of tissues from the forestomach to detect the neuroendocrine cell markers SYP and NNE, glial cell markers GFAP and VIM, and peptidergic innervation markers NPY and VIP. The tissue sections were deparaffinized and hydrated by their immersion in descending concentration of ethanol (100%, 96%, 80%, and 70%) and distilled water. For antigen retrieval, the sections were microwaved in 0.01 M buffer citrate solution (pH 6) for 5 min at 800 watts. Endogenous peroxidase activity was blocked by incubation with 0.5% hydrogen peroxide for 30 min at room temperature. Non-specific binding was blocked by incubation in 1% normal goat serum (X0907; Dako, USA) for 30 min at room temperature.
The samples were then incubated for 30 min at room temperature with the following primary antibodies: mouse monoclonal anti-SPY (1 : 10 dilution, MA1-35810; Thermo Scientific, USA), rabbit polyclonal anti-NNE (1 : 50 dilution, 6880-0410; AbD Serotec, USA); polyclonal anti-GFAP (1 : 200 dilution, RB-087-R7; Thermo Scientific); mouse monoclonal anti-VIM (1 : 50 dilution, MS-129-R7; Thermo Scientific); rabbit polyclonal anti-NPY (1 : 50 dilution, PA1-41576; Thermo Scientific), and rabbit polyclonal anti-VIP (1 : 50 dilution, 9535-0204; AbD Serotec). The sections were then incubated with HRP-conjugated polymer (1 : 50 UltraVision ONE HRP Polymer, TL-015-PHJ; Thermo Scientific) for 30 min at room temperature in the dark. Antibody binding was visualized by incubation with diaminobenzidine (40 µL 1drop DAB Plus Chromogen TA-001-HCX to 2mL DAB Plus Substrate TA-015-HSX; Thermo Scientific) for 5~15 min depending on the desired staining intensity at room temperature. Finally, the sections were counterstained with Mayer's hematoxylin (S3309; Dako). Staining specificity was evaluated by performing control experiments in which the primary antiserum was replaced with PBS.
Immunolabeled sections were analyzed using the Nis-Element Br 2.30 software package (Nis-Element Basic Research 2.30, USA). The stained surface was examined to evaluate various tissue strata (epithelium, lamina propria and submucosa, tunica muscularis, serosa, and myenteric plexus) and the whole wall. Optimal intervals were performed statistically and four categories of immunoreactivity intensity were established [17]: no immunoreactivity, no surface staining; low immunoreactivity, stained surface less than 200 µm2 in size; moderate immunoreactivity, stained surface between 200 and 400 µm2; and intense immunoreactivity, stained surface over 400 µm2.
Measurements expressed in µm2 are presented as the mean ± standard error (SE). Data were subjected to an analysis of variance (ANOVA). Wherever significant differences were found by the ANOVA, a post-hoc (Tukey's) test was performed to identify significant differences between tissue strata and groups. A p value < 0.05 was considered significant. The SPSS.19 statistical software package licensed to Extremadura University was used for this purpose.
At 35 days (CRL 3 cm, 23% gestation), the rumen, reticulum, and omasum became separate compartments of the primitive gastric tube. The wall consisted of three layers: an internal stratified epithelium layer, a middle layer of pluripotencial blastemic tissue, and an external layer or serosa. At 38 days (CRL 4.3 cm, 25% gestation), rudimentary primary omasal laminae appeared as small protrusion from the omasal wall.
The stratified epithelium was divided into two bands: a stratum basal formed by two~three layers of germinal cells with basophilic cytoplasm and a stratum granulosum consisting of five~six layers of globose cells with a light cytoplasm. At 46 days (CRL 6 cm, 30% gestation), small papilliform projections were observed in the ruminal wall that extended to rudimentary pillars. At 50 days (CRL 7.7 cm, 33% gestation), the lamina propria and submucosa arose from pluripotencial blastemic tissue. By this stage, the tunica muscularis was distinguishable from pluripopotencial blastemic tissue as a layer of longitudinal-arranged myoblasts. Moreover, secondary omasal laminae began to appear as small elevations between primary laminae. Primary omasal laminae were larger and more numerous at this stage of gestation.
The wall of the three gastric compartments was formed by four layers: mucosa (composed of an epithelium and lamina propria), submucosa, tunica muscularis, and serosa. At 53 days (CRL 9 cm, 35% gestation) slight evaginations of the epithelium were visible and formed rudimentary ruminal papillae. The lamina propria and submucosa were composed of fibroblast-rich connective tissue interspersed among ground substance. No separation was apparent between the two layers. The tunica muscularis was formed by two layers: an inner circular layer and an outer longitudinal layer. At 59 days (CRL 10 cm, 38% gestation), primary reticular crests were visible and tertiary omasal laminae were observed in the spaces between the primary and secondary laminae. Quaternary laminae were also visible at 64 days (CRL 13.5 cm, 43% gestation). At this stage, the muscularis mucosae was observed at the center of the omasal laminae and arose from the inner circular layer of smooth muscle fibers of the tunica muscularis. At 70 days (CRL 15 cm, 47% gestation), conical papillae started to rise from the surface of primary omasal laminae. The serosa consisted of a subserosa composed of loose fibrous connective tissue underlying a mesothelial layer of flat cells.
In this group, the stratified epithelium contained four layers. The stratum basale was formed by basophilic cells. The stratum granulosum contained polyhedral cells with lightly stained cytoplasm. The stratum spinosum was superficial to the stratum granulosum and stratum corneum formed by a single layer of flat cells.
At 76 days (CRL 18 cm, 50% gestation), ruminal papillae were more developed than previously observed. These structures appeared as small elevations of the basal area rising towards the ruminal lumen. At this gestational age, conical papillae were found in the secondary laminae in the omasum. At 87 days (CRL 22 cm, 61% gestation), the lateral surface of the primary reticular crest was studded with small corneum papillae. At 101 days (CRL 27.5 cm, 67% gestation), a secondary reticular crest was visible. At this stage, a layer of smooth muscle fibers was observed in the uppermost area of the primary reticular crest that formed the muscularis mucosae.
The ruminal, reticular, and omasum mucosa were covered by a stratified epithelium that was divided into a stratrum basale, granulosum, spinosum, and corneum. The lamina propria and submucosa contained fibroblasts and collagen fibers among ground substance. There was no clear separation between the submucosa and the lamina propria. The muscularis mucosa was fully developed and occupied the center of the omasal laminea and top of the reticular crests. The tunica muscularis contained two layers of smooth muscle fibers in an inner circular layer and outer longitudinal layers. Ruminal papillae were both longer and thicker, and reached the apical third of the epithelium. The reticular crests had two growth patterns when forming reticular cells: longitudinal and transversal. In the omasum, four orders of omasal laminae of varying thickness were apparent. All were studded with numerous conical papillae. Each gastric compartment was lined by an external serosa formed by a subserosa of connective tissue and overlying mesothelium.
Immunoreactivity specific for SYP, NNE, GFAP, VIM, NPY, and VIP was observed in all forestomachs of the embryos and fetuses. The total immunostained areas (µm2) in the rumen, reticulum, and omasum are shown in Tables 1, 2, and 3, respectively. The staining intensity for each factor in each stratum of the goat forestomach is presented in Fig. 1. Distribution of the different markers in the rumen, reticulum, and omasum is shown in Figs. 2, 3, and 4, respectively.
SPY immunoreactivity was observed at 53 days (CRL 9 cm, 35% gestation) and was distributed unevenly. Staining was more intense in the tunica muscularis than the lamina propria-submucosa (Fig. 2A).
Positive staining was observed at 53 days (CRL 9 cm, 35% gestation) in the lamina propria-submucosa, tunica muscularis, serosa, and myenteric plexus. The immunostained surface was larger in the tunica muscularis than the lamina propria-submucosa (Fig. 3A).
SPY expression was detected at 53 days (CRL 9 cm, 35% gestation) in the lamina propria and submucosa, tunica muscularis, and myenteric plexus (Fig. 4A). At 113 days (CRL 33 cm, 75% gestation), small stained areas were observed in the connective tissue of the omasal laminae.
NNE immunoreactivity was observed at 64 days (CRL 13.5 cm, 43% gestation) in all strata of the ruminal wall although the distribution patterns differed between strata (Fig. 2B).
Positive staining for NNE was detected at 64 days (CRL 13.5 cm, 43% gestation) in the lamina propria-submucosa, tunica muscularis, serosa, myenteric plexuses, and epithelium. Staining in the epithelium was significantly more intense during the later stages of prenatal development (Fig. 3B).
NNE expression was observed at 64 days (CRL 13.5 cm, 43% gestation) in the lamina propria-submucosa, tunica muscularis, serosa, epithelium, and myenteric plexuses. The epithelium was the most heavily stained layer in all groups (Fig. 4B).
GFAP-specific immunoreactivity was clearly observed at 68 days (CRL 15 cm, 45% gestation) in the lamina propria-submucosa, tunica muscularis, serosa, and myenteric plexuses. The most intense staining was found in the myenteric plexuses (Fig. 2C).
Positive staining for GFAP was detected after 64 days (CRL 13.5 cm, 43% gestation) in the lamina propria-submucosa, tunica muscularis, serosa, and myenteric plexuses (Fig. 3C). A moderate intensity of immunoreactivity was observed in the myenteric plexus as prenatal development progressed.
GFAP immunoreactivity was detected at 64 days (CRL 13.5 cm, 43% gestation) in the lamina propria-submucosa, tunica muscularis, and myenteric plexuses. The greatest staining intensity was observed in the myenteric plexuses (Fig. 4C). GFAP-immunoreactivity (Ir) was occasionally detected in the connective tissue of the omasal laminae.
VIM expression was detected at 39 days (CRL 4.4 cm, 25% gestation) in mesenchymal cells and the serosa (Fig. 2D). At 53 days, immunoreactivity was restricted to the lamina propria-submucosa, tunica muscularis, serosa, and myenteric plexuses. Staining intensity decreased in each strata.
Positive staining for VIM was observed at 39 days (CRL 4.4 cm, 25% gestation) in mesenchymal cells and the serosa (Fig. 3D). From 50 days (CRL 7.7cm, 33% gestation), a moderate immunoreactivity was observed in lamina propria-submucosa, tunica muscularis and the myenteric plexus. A low immunoreactivity was detected in serosa from 64 days (CRL 13.5 cm, 43% gestation).
VIM-positive glial cells were observed at 39 days (CRL 4.4 cm, 25% gestation) in pluripotential blastemic tissue and serosa (Fig. 4D). By 53 days (CRL 9 cm, 35% gestation), moderate immunoreactivity was observed in the lamina propria-submucosa and serosa and to a lesser degree in the tunica muscularis. A low level of inmmunorreactivity was also found in the myenteric plexus.
NPY-positive staining was detected at 113 days (CRL 33 cm, 75% gestation). Immunoreactivity was distributed in the lamina propia-submucosa and tunica muscularis. The myenteric plexus contained a moderate level of immunoreactivity (Fig. 2E).
NPY expression was first observed at 75 days (CRL 17.5 cm, 50% gestation) in the lamina propria-submucosa and tunica muscularis. A moderate degree of immunoreactivity was found in the myenteric plexus. Stained areas were observed in the connective tissue of the crest ribs (Fig. 3E).
NPY immunoreactivity was detected at 95 days (CRL 20 cm, 63% gestation) in all tissue layers of the omasal wall except for the epithelium. There was no significant difference in staining intensity between groups IV and V. NPY staining was also observed in the connective tissue of the omasal laminae (Fig. 4E).
VIP expression was detected at 113 days (CRL 33 cm, 75% gestation) in the lamina propria-submucosa, tunica muscularis, and myenteric plexus (Fig. 2F).
VIP-positive staining was observed at 75 days (CRL 17.5 cm, 50% gestation) in the lamina propria-submucosa, tunica muscularis, serosa, and myenteric plexus. Significant differences were found between groups IV and V (Fig. 3F).
VIP immunoreactivity was detected at 95 days (CRL 20 cm, 63% gestation) in the lamina propiasubmucosa, tunica muscularis, serosa, and myenteric plexus. Small stained areas were found in the connective tissue of the omasal laminae (Fig. 4F).
In this study, we charted the spatial and temporal expression of neuroendocrine cell, glial cell, and peptidergic innervation markers in the goat forestomach. SPY-positive neuroendocrine cells were observed at 53 days of prenatal development (35% gestation). Similar findings have been previously noted in the goat rumen, omasum and reticulum [11,12,13]. Similar results have also been reported for other ruminant species. In deer, for example, neuroendocrine cells have been detected at 97 days (36% gestation) in the rumen and reticulum [9,10], and at an earlier stage of 67 days (26% gestation) in the omasum [28]. In contrast, neuroendocrine cells are not found in sheep until 81 days (54% gestation) [6,7,29]. In the current investigation, SPY immunoreactivity was observed predominantly in the tunica muscularis and myenteric plexuses. However, a less immunoreactivity was found in the lamina propria-submucosa and serosa. Staining intensity increased significantly with gestational age as was previously reported in sheep [3].
NNE-positive neuroendocrine cells were detected at a later stage than SPY-positive cells. The staining distribution and intensity patterns for the two factors were similar except that NNE staining was prominent in the epithelium of all forestomach compartments. This finding seems to suggest that these neuroendocrine cells belong to the diffuse neuroendocrine system located in the gastrointestinal epithelium [23]. Diffuse neuroendocrine system cells in the digestive tract are thought to arise from the same precursor cell as a different type of epithelial cell [32]. These neuroendocrine cells originating in the embryonic neural crest migrate to the gastrointestinal epithelium during the course of prenatal development [24].
GFAP-positive glial cells were detected at 68 days (45% gestation) in the rumen, and at 64 days (43% gestation) in reticulum and omasum. A similar finding was reported in the rumen and reticulum of sheep and deer at 112 days (75% gestation) and 142 days (50% gestation), respectively [6,7]. However, glial cells have been observed at earlier stages in the omasum of these two species [28,29]. GFAP-positive staining was visible in the lamina propria-submucosa, tunica muscularis, and serosa, and was particularly prominent in the myenteric plexuses. The presence of glial cells in the myenteric plexuses and submucosa has also been reported in sheep [38] and cows [34]. In other species including rats [22] and cats [18], GFAP-positive cells have been found in the ganglionate plexuses. Glial cells are similar in structure and function to astrocytes of the central nervous system, and play a key role in controlling gastrointestinal functions and protecting enteric neurons [1].
Glial cells were also detected by VIM staining. VIM positivity was observed in all forestomach compartments at 39 days of gestation. This finding indicated that VIM is an earlier glial cell marker than GFAP and corresponds to observations reported during prenatal development of the sheep pineal gland [8] as well as the forestomach of sheep and deer [6,7,29]. At 39 days (25% gestation), VIM immunoreactivity was detected in pluripotential blastemic tissue and serosa. From 50 days of gestation, this immunoreactivity was distributed in the different tissue layers that were already differentiated. VIM positivity was observed in the same strata as GFAP staining.
Peptidergic innervation markers detected in the goat forestomach have also been reported in lambs [15]. In the present investigation, these factors were first observed in the reticulum and omasum, and later in the rumen. Similar findings have been reported for red deer in which these markers were detected at 142 days (50% gestation) in the reticulum [7,10] and omasum [28,29], but not in the rumen until the perinatal stages [6,9]. Positive staining for NPY and VIP had a similar pattern. For both makers, the myenteric plexuses contained the greatest staining intensity. In lambs, NYP-positive staining in the myenteric plexuses has low to moderate intensity while that for VIP is more intense [15]. In contrast, a high density immunoreactive for VIP is most marked in the smooth muscle layers of the reticular groove, reticulum, and rumen of cattle [17], and omasum of sheep [38]. Peptide distribution observed in both the present investigation and earlier studies [16,17] indicates that peptide-containing nerve fibers are mainly intrinsic in origin and derived from the intramural ganglia of the forestomach wall.
In conclusion, glial cell, neuroendocrine cell, and peptidergic innervation markers were detected in the goat forestomach during prenatal development. Differentiation of these cell types took place concurrently with the differentiation of tissue strata. These findings indicate a possible function of the fetal goat forestomach during preparation for postnatal function. Compared to other ruminant species, neuroendocrine cells, glial cells and peptidergic innervations markers as NPY and VIP were detected earlier in goats than sheep, but around the same stage as deer.
Figures and Tables
Fig. 1
Immunoreactivity for each antibody in the different layers of rumen, reticulum, and omasum in goats.

Fig. 2
Distribution of immunoreactivity in the goat rumen. (A) Synaptophysin (SPY)-positive staining in the tunica muscularis and myenteric plexus (arrow) in a section of rumen wall at 64 days (43% gestation). (B) Non-neuronal enolase (NNE) immunoreactivity in the epithelial layer (arrow), lamina propria-submucosa, tunica muscularis, and myenteric plexus (arrow) at 64 days (43% gestation). (C) Glial fibrillary acidic protein (GFAP)-positive staining at 64 days (43% gestation) in the lamina propria-submucosa, tunica muscularis, serosa, and myenteric plexus, (arrows) and within ruminal papillae. (D) Vimentin (VIM)-specific immunostaining in the mesenchymal cells and serosa of the ruminal wall at 39 days (25% gestation). (E) Neuropeptide Y (NPY)-positive staining in the lamina propria-submucosa, tunica muscularis, and myenteric plexus (arrows) at 113 days (75% gestation). (F) Vasoactive intestinal peptide (VIP)-specific staining at 113 days (75% gestation) in the lamina propria-submucosa. Intense immunostaining was observed in the tunica muscularis and myenteric plexus (arrows). Scale bars = 20 µm (D), 25 µm (A, B, E, and F), or 30 µm (C).
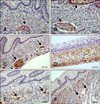
Fig. 3
Distribution of immunoreactivity in the goat reticulum. (A) SYN-positive staining in the lamina propria-submucosa, tunica muscularis, serosa, and myenteric plexus (arrow) at 64 days (43% gestation). (B) NNE-specific immunoreactivity visible in the epithelium (arrow), lamina propria-submucosa, tunica muscularis, serosa, and myenteric plexus (arrow) at 69 days (46% gestation). (C) GFAP-positive staining in the lamina propria-submucosa, tunica muscularis, and myenteric plexus (arrow) at 64 days (43% gestation). (D) VIM-specific immunostaining in the pluripotential blastemic tissue layer and serosa at 39 days (25% gestation). (E) NPY-positive staining in the lamina propria-submucosa, tunica muscularis, and myenteric plexus (arrow) at 75 days (50% gestation). (F) VIP-specific staining in the lamina propria-submucosa, tunica muscularis, and myenteric plexus (arrows) at 75 days (50% gestation). Scale bars = 25 µm (A, C, D, and E), 20 µm (B), or 30 µm (F).
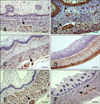
Fig. 4
Distribution of immunoreactivity in the goat omasum. (A) SYN-positive staining in the lamina propria-submucosa, tunica muscularis, and myenteric plexus in the omasal wall at 75 days (50% gestation). (B) NNE-specific immunoreactivity in the epithelial layer, lamina propria-submucosa, and tunica muscularis at 64 days (75% gestation). (C) GFAP-positive staining within the myenteric plexus (arrow), lamina propria-submucosa, tunica muscularis, and serosa at 69 days (46% gestation). (D) VIM-specific immunostaining in the pluripotential blastemic tissue layer and serosa at 39 days (25% gestation). (E) NPY-positive staining in the lamina propria-submucosa, tunica muscularis, and myenteric plexus. An immunostained surface was visible in the connective tissue of the omasal laminae at 95 days (63% gestation). (F) VIP-positive staining in the connective tissue and tunica muscularis of the omasal laminae at 113 days (75% gestation). Scale bar = 25 µm (A, C, and E) or 20 µm (B, D, and F).
Table 1
Total stained surface area (µm2) in the goat rumen of the different gestational groups

Group I (CRL 1.5~4.3 cm, 26~38 days: 1~25% gestation), Group II (CRL 4.4~8 cm, 39~52 days: 25~35% gestation), Group III (CRL 9~17.5 cm, 53~75 days: 35-50% gestation), Group IV (CRL 18~32 cm, 76~112 days: 50~75% gestation), Group V (CRL 33~47 cm, 113~150 days: 75~100% gestation). SYP: synaptophysin, NNE: non-neuronal enolase, GFAP: glial fibrillary acidic protein, VIM: vimentin, NPY: neuropeptide Y, VIP: vasoactive intestinal peptide.
Acknowledgments
This research was supported by the Extremadura Regional Government and the European Social Fund, Spain (project PRE 08055). The authors thank Ms. Pilar Parra of the Histology Section at the Veterinary Faculty of Extremadura (Spain) for providing technical assistance with this project.
References
1. Abdo H, Derkinderen P, Gomes P, Chevalier J, Aubert P, Masson D, Galmiche JP, Vanden Berghe P, Neunlist M, Lardeux B. Enteric glial cells protect neurons from oxidative stress in part via reduced glutathione. FASEB J. 2010; 24:1082–1094.

2. Calingasan NY, Kitamura N, Yamada J, Oomori Y, Yamashita T. Immunocytochemical study of the gastroenteropancreatic endocrine cells of the sheep. Acta Anat. 1984; 118:171–180.

3. Ceccarelli P, Pedini V, Gargiulo AM. Enteroendocrine cells in sheep fetuses. Small Rumin Res. 1991; 6:85–93.

4. Deveney CW, Way WL. Regulatory peptides of the gut. In : Greenspan FS, Forsham PH, editors. Basic and Clinical Endocrinology. Asian ed. Singapore: Maruzen;1983. p. 479–499.
5. Evans HE, Sack WO. Prenatal development of domestic and laboratory mammals: growth curves, external features and selected references. Zentralbl Veterinarmed C. 1973; 2:11–45.

6. Franco A, Masot AJ, Redondo E. Ontogenesis of the rumen: a comparative analysis of the merino sheep and Iberian red deer. Anim Sci J. 2011; 82:107–116.

7. Franco A, Masot J, García A, Redondo E. Ontogenesis of the reticulum with special reference to neuroendocrine and glial cells: a comparative analysis of the merino sheep and Iberian red deer. Anat Histol Embryol. 2012; 41:362–373.

8. Franco A, Regodon S, Masot AJ, Redondo E. A combined immunohistochemical and electron microscopic study of the second cell type in the developing sheep pineal gland. J Pineal Res. 1997; 22:130–136.

9. Franco AJ, Masot AJ, Aguado MC, Gómez L, Redondo E. Morphometric and immunohistochemical study of the rumen of red deer during prenatal development. J Anat. 2004; 204:501–513.

10. Franco AJ, Redondo E, Masot AJ. Morphometric and immunohistochemical study of the reticulum of red deer during prenatal development. J Anat. 2004; 205:277–289.

11. Garcia A, Masot J, Franco A, Gazquez A, Redondo E. Histomorphometric and immunohistochemical study of the goat omasum during prenatal development. Histol Histopathol. 2013; 28:737–748.
12. Garcia A, Masot J, Franco A, Gazquez A, Redondo E. Histomorphometric and immunohistochemical study of the goat reticulum during prenatal development. Histol Histopathol. 2013; 28:1369–1381.
13. García A, Masot J, Franco A, Gázquez A, Redondo E. Histomorphometric and immunohistochemical study of the goat rumen during prenatal development. Anat Rec (Hoboken). 2012; 295:776–785.

14. Gershon MD, Kirchgessner AL, Wade PR. Functional anatomy of the enteric nervous system. In : Johnson LR, editor. Physiology of the Gastrointestinal Tract. 3rd ed. New York: Raven Press;1994. p. 381–422.
15. Groenewald HB. Neuropeptides in the myenteric ganglia and nerve fibres of the forestomach and abomasum of grey, white and black Karakul lambs. Onderstepoort J Vet Res. 1994; 61:207–213.
16. Kitamura N, Yamada J, Yamamoto Y, Yamashita J. Substance P-immunoreactive neurons of the bovine forestomach mucosa: their presumptive role in a sensory mechanism. Arch Histol Cytol. 1993; 56:399–410.

17. Kitamura N, Yamada J, Yamashita T. Immunohistochemical study on the distribution of neuron-specific enolase- and peptide-containing nerves in the reticulorumen and the reticular groove of cattle. J Comp Neurol. 1986; 248:223–234.

18. Kleinschmidt S, Nolte I, Hewicher-Trautwein M. Structural and functional components of the feline enteric nervous system. Anat Histol Embryol. 2011; 40:450–456.

19. Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980; 283:249–256.

20. Lombardi G. Optimum management and quality pastures for sheep and goat in mountain areas. Options Méditerranéennes. 2005; 67:19–29.
21. Münnich J, Gäbel G, Pfannkuche H. Intrinsic ruminal innervation in ruminants of different feeding types. J Anat. 2008; 213:442–451.

22. Nada O, Kawana T. Immunohistochemical identification of supportive cell types in the enteric nervous system of the rat colon and rectum. Cell Tissue Res. 1988; 251:523–529.

23. Pan QS, Fang ZP, Huang FJ. Identification, lozalization and morphology of APUD cell in gastroenteropan creatic system of stomach-containing teleosts. World J Gastroenterol. 2000; 6:842–847.

24. Pearse AGE, Takor TT. Neuroendocrine embryology and the APUD concept. Clin Endocrinol (Oxf). 1976; 5:Suppl. 229S–244S.

25. Pfannkuche H, Schellhorn C, Schemann M, Gäbel G. Intrinsic innervation patterns of the smooth muscle in the rumen and reticulum of lambs. J Anat. 2004; 204:293–299.

26. Pfannkuche H, Schellhorn C, Schemann M, Gäbel G. Reticular groove and reticulum are innervated by myenteric neurons with different neurochemical codes. Anat Rec A Discov Mol Cell Evol Biol. 2003; 274:917–922.

27. Pfannkuche H, Schemann M, Gäbel G. Ruminal muscle of sheep is innervated by non-polarized pathways of cholinergic and nitrergic myenteric neurones. Cell Tissue Res. 2002; 309:347–354.

28. Redondo E, Franco AJ, Masot AJ. Morphometric and immunohistochemical study of the omasum of red deer during prenatal development. J Anat. 2005; 206:543–555.

29. Redondo E, Masot J, García A, Franco A. Ontogenesis of the omasum: a comparative analysis of the merino sheep and Iberian red deer. Histol Histopathol. 2011; 26:1135–1144.
30. Ruckebusch Y. Gastrointestinal motor functions in ruminants. In : Schultz SG, Wood JD, Rauner BB, editors. Handbook of Physiology. The Gastrointestinal System. New York: Oxoford University Press;1989. Vol. 1:p. 1225–1283.
31. Schummer A, Nickel R. Lehrbuch der Anatomie der Haustiere: Eingeweide. 3rd ed. Parey: Berlin;1975. Vol. 2:p. 149–173.
32. Sidhu GS. The endodermal origin of digestive and respiratory tract APUD cells. Histopathologic evidence and a review of the literature. Am J Pathol. 1979; 96:5–20.
33. Sivachelvan MN, Ali MG, Chibuzo GA. Foetal age estimation in sheep and goats. Small Rumin Res. 1996; 19:69–76.

34. Teixeira AF, Wedel T, Krammer HJ, Kühnel W. Structural differences of the enteric nervous system in the cattle forestomach revealed by whole mount immunohistochemistry. Ann Anat. 1998; 180:393–400.

35. Titchen DA. Nervous control of motility of the forestomach of ruminant. In : Heidel W, Code CF, editors. Handbook of Physiology: Alimentary Canal. Baltimore: Waverly Press;1968. Vol. 5:p. 2705–2724.
36. Von Boyen GBT, Steinkamp M, Reinshagen M, Schäfer KH, Adler G, Kirsch J. Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut. 2004; 53:222–228.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download