Abstract
This study was conducted to evaluate an adapter-modified Ussing chamber for assessment of transport physiology in endoscopically obtained duodenal biopsies from healthy cats and dogs, as well as dogs with chronic enteropathies. 17 duodenal biopsies from five cats and 51 duodenal biopsies from 13 dogs were obtained. Samples were transferred into an adapter-modified Ussing chamber and sequentially exposed to various absorbagogues and secretagogues. Overall, 78.6% of duodenal samples obtained from cats responded to at least one compound. In duodenal biopsies obtained from dogs, the rate of overall response ranged from 87.5% (healthy individuals; n = 8), to 63.6% (animals exhibiting clinical signs of gastrointestinal disease and histopathological unremarkable duodenum; n = 15), and 32.1% (animals exhibiting clinical signs of gastrointestinal diseases and moderate to severe histopathological lesions; n = 28). Detailed information regarding the magnitude and duration of the response are provided. The adapter-modified Ussing chamber enables investigation of the absorptive and secretory capacity of endoscopically obtained duodenal biopsies from cats and dogs and has the potential to become a valuable research tool. The response of samples was correlated with histopathological findings.
Chronic enteropathies occur frequently in both cats and dogs, and diagnosis of the underlying cause can be challenging owing to the non-specific clinical presentation and lack of accurate diagnostic markers. To arrive at the correct diagnosis, diseases with similar clinical signs, such as idiopathic inflammatory bowel disease (IBD), food allergy, infection with pathogenic enteric bacteria, parasitic infestation, or others must be differentiated, which requires a systematic and time consuming work-up of the affected patient [1,9,17,19].
Ussing chambers allow assessment of the transport physiology of the intestinal mucosa. In humans, gastrointestinal disorders such as food allergy or cystic fibrosis can be characterized by evaluation of surgically-collected biopsies using the Ussing chamber [4,7]. Evaluation of intestinal transport physiology with a Ussing chamber might also be beneficial as a research and/or diagnostic tool for gastrointestinal disorders in dogs and/or cats. We previously demonstrated that an adapter-modified Ussing chamber could be used for evaluation of transport physiology of colonic biopsies [31]. The present study was conducted to investigate the use of the adapter-modified Ussing chamber for evaluation of intestinal mucosal transport in endoscopically collected biopsies from the duodenum of dogs and cats, as well as to assess duodenal transport physiology of healthy dogs and dogs with gastrointestinal diseases using this technology.
An adapter-modified Ussing chamber that was described previously (Ussing system CH8; World Precision Instruments, USA; Fig. 1) was used for this study [31]. The centrally placed adapter disc enables investigation of tissue samples covering a size of 5 mm2, which is the approximate size of endoscopically collected intestinal biopsy samples (eMachineShop, USA). Air suction at 400 mmHg was applied through a cannula passed through a side hole into the body of the disc, enabling sealing and stabilization of the intestinal biopsy sample in the center aperture. The biopsy sample was surrounded by oxygenated physiologic buffer (10 mM HEPES, 0.3 mM Na2HPO4, 0.4 mM NaH2PO4, 1.0 mM MgCl2, 1.3 mM CaCl2, 4.7 mM KCl, 105 mM NaCl, 20.2 mM NaCO3, pH 7.4) that was maintained at 37℃ and continuously oxygenated with 95% O2/5% CO2.
An Olympus GIF-160 gastroscope with a 2.8 mm working channel (Olympus, USA) was used to collect biopsy samples from each animal enrolled in the study. Biopsies were taken with standard biopsy forceps (Olympus FB-36 K-1, diameter 2.8; Olympus). The morphology of the biopsies was first evaluated under a microscope, after which the thickest and most intact biopsies were transferred into the Ussing chambers within 10~15 min of collection. Other intact samples were fixed for histopathological evaluation. After completing the experiment, the tested biopsy samples were also fixed for histopathologic analysis. However, after being held in a water bath in the Ussing chamber for 220 min, some samples were destroyed and could not be preserved. In general, we used three to four samples that had been in the Ussing chamber and two control samples for histopathologic analysis.
The electrical circuit of the Ussing chamber was established by four Silver/Silver-Chloride electrodes (EK1; World Precision Instruments, USA). Three percent agarose bridges in 3 M KCl ensured an electrical circuit between the buffer solution in the Ussing Chamber and the electrodes. The electrodes were connected to a preamplifier, operated by an amplifier (DVC-1000; World Precision Instruments), and recorded (iWorx, USA). After transfer of a biopsy sample into the Ussing chamber, an equilibration time of 5~10 min was allowed. Subsequently, the tissue was short-circuited, cycling every 10 sec in current clamp mode to enable measurement of the short circuit current (Isc) and conductance (G). While the voltage was maintained at zero, Isc reflected the intensity of the active ion transport. By comparing the current before application of a chemical compound to the maximum current after exposure to a chemical compound (ΔIsc), the specific effect of the chemical was assessed. This information reflects the active tissue response to a specific substance. The time from the onset of ΔIsc until a maximum Isc value was also calculated (Δt).
After mounting the loaded discs, each half of the water reservoir was concurrently filled with 10 mL of buffer solution. The buffer solution surrounding the lamina submucosa (serosal side) was modified by adding 10 mM glucose, which was designed to provide nutritional support to the intestinal cells. To maintain osmotic balance between the two sides of the chamber, 10 mM sorbitol was added to the buffer surrounding the enterocytes (mucosal solution). The absorptive activity in each biopsy sample was investigated by adding 40 mM glucose followed by 500 µM phloridzin to the mucosal bathing solution. While glucose is taken up by sodium-glucose co-transporter 1 (SGLT-1) in combination with positively charged sodium, glucose uptake can be visualized by a resulting change in Isc. Phloridzin is a competitive inhibitor of SGLT-1 transporter that inhibits the previously induced ΔIsc and clearly identifies the pathway of glucose uptake. The sequential and continuous addition of 200 µM histamine, 200 µM 5-hydroxytryptamine (5-HT), 4 µM prostaglandin (PGE2), and 5 µM forskolin to the serosal buffer solution allowed evaluation of the secretory response of the biopsy sample. These secretagogues affect different pathways, but their final action results in chloride secretion. The loss of negative charge across the membrane can again be visualized by a change in Isc. All substances were added as soon as the original baseline current was reached, or at least a steady state level was obtained. In cases of a sample being non-responsive, the appropriate time for the usual tissue response was allowed to pass before adding the next chemical compound. To evaluate biopsy viability at the end of the experiment, another 40 mM glucose followed by 600 µM ouabain was applied to the mucosal or serosal buffer solution, respectively.
Seventeen duodenal biopsy samples were collected endoscopically from five cats and 51 duodenal biopsy samples were collected endoscopically from 13 dogs at Texas A & M University. Two healthy dogs underwent anesthesia for reasons unrelated to gastrointestinal disease. For those two dogs, no history of gastrointestinal symptoms had been reported and no medication, other than preventative medication against flea and heartworm infestation, had been administered. All other dogs and cats showed a variety of gastrointestinal symptoms, including vomiting, diarrhea, and/or anorexia, and received various medications. Histopathological diagnosis of all biopsy samples was performed according to the histopathological standards of gastrointestinal inflammation of endoscopic biopsy samples published by the World Small Animal Veterinary Association Gastrointestinal Standardization group (WSAVA) by a board certified pathologist blinded to the results of this study [9]. To investigate transport physiology in animals of varying health status, data describing dogs were grouped according to the WSAVA grading system in combination with the occurrence of clinical signs. Group 1 consisted of two healthy dogs with no history of gastrointestinal symptoms and unremarkable histopathological biopsy samples (number of biopsies evaluated by the Ussing chamber n = 8). Group 2 consisted of four dogs with clinical signs of gastrointestinal disease, but unremarkable histopathological biopsy samples (number of biopsies evaluated by the Ussing chamber n = 15). Group 3 consisted of seven dogs with clinical signs of gastrointestinal disease and moderate to severe histopathological lesions (number of biopsies evaluated by the Ussing chamber n = 28). The severities of histopathological changes of the biopsy samples were grouped based on the highest severity in any of the parameters assessed. Animals for which the exact age was not known were assumed to be born on January 1st of their reported year of birth. Detailed information regarding the animals enrolled into this study is listed in Table 1.
The protocol for this study was reviewed and approved by the Clinical Research Review Committee of the Texas A&M Veterinary Medical Center. All owners gave their informed consent for enrollment of their animal into the study.
Data were evaluated for normal distribution by the D'Agostino normality test and results (ΔIsc, G) were expressed as medians (ranges), or mean ± standard deviation (SD), where appropriate. Data were analyzed using a commercial software package (GraphPad Prism 5; GraphPad Software, USA).
The overall mean (± SD) baseline conductance of duodenal biopsy samples obtained from cats and dogs was 61.7 (± 28.7) mS/cm2(resistance: 20.3 ± 10.8 Ω·cm2, n = 14) and 89.9 (± 71.7) mS/cm2(resistance: 17.3 ± 10.3 Ω· cm2, n = 44), respectively. The mean conductances of groups 1, 2, and 3 were 48.9 (± 15.2), 99.9 (± 77.5) and 96.7 (± 76.4) mS/cm2, respectively. The total duration of the experiments performed on biopsy samples obtained from cats varied between 40.3 and 220.2 min (median: 82.3 min). The results from experiments with these duodenal biopsy samples obtained from cats are summarized in Table 2. The median duration for experiments performed on biopsy samples obtained from dogs was 90.9 min (range: 75.1~153.0 min). A total of 19 biopsies from dogs did not respond to any substance. Three of those biopsy samples were included in group 2, while 16 samples were in group 3. All results, as well as the results for individual groups, are displayed in Table 3.
Of 17 biopsy samples tested, two duodenal biopsy samples obtained from cats responded to stimulation with 40 mM glucose. Thus, the response rate of duodenal biopsy samples obtained from cats to glucose was poor (11.8%). However, immediately after transferring glucose into the mucosal bathing solution, the current measured for the two responding biopsy samples increased by 24 µA/cm2 and 32 µA/cm2, respectively. The biopsy sample with the highest value took 111.4 sec to reach its maximum value, while the other biopsy sample took only 68.4 sec to reach its maximum value. The short circuit current remained on a plateau until it was abolished by the addition of phloridzin. Both of these biopsy samples were collected from the same cat. At least one duodenal biopsy sample from each cat responded to 500 µM phloridzin. Overall, 12 of 17 (70.6%) exposed biopsy samples obtained from cats responded to phloridzin. No correlation between time to maximum effect and level of ΔIsc was observed (Table 2).
Out of 51 biopsy samples, 21 showed a response demonstrated by an increase in their short circuit current. The median amplitude of those responding samples was 34 µA/cm2, and ranged from 6 to 170 µA/cm2. While 75% of the biopsy samples from group 1 responded to glucose, only 53.3% and 25% of biopsies of group 2 and group 3 showed an adequate response, respectively (Table 3). There was a large degree of variation in the baseline current, Δt, as well as ΔIsc, regardless of the prevalence of histopathological lesions and/or clinical symptoms of the patients.
Overall, 51 duodenal biopsy samples obtained from dogs were exposed to 500 µM phloridzin. The ΔIsc of 25 biopsy samples decreased immediately (range: 14~168 µA/cm2; median value: 32 µA/cm2). Similar to glucose exposure, a higher percentage of biopsy samples from group 1 (87.5%) than from group 2 (60%) and group 3 (32.1%) responded to phloridzin (Table 3).
Of the 17 tested duodenal biopsy samples, eight responded to the application of 200 µM histamine. In five biopsy samples (ΔIsc ranging from 4 to 8 µA/cm2), the current rose for a few seconds and, after a slow but steady fall, reached baseline levels after 56.8~ 771.5 sec. Comparatively, the current of three other biopsy samples (ΔIsc ranging from 26 to 30 µA/cm2) resulted in a biphasic graph. After an immediate sharp increase in current, the values kept increasing slowly until reaching a maximum value after 44.7~54.0 sec. Isc then slowly returned to baseline values after 187.1~203.0 sec. The three biopsy samples were from the same cat (Table 2).
Only five of 51 biopsy samples showed a response to histamine application. Two of these were collected from dogs of group 1, while three were from dogs of group 3 (Table 3). The curve of the short circuit current change was hill-shaped, with a steep rise and a slow and long decline. The Isc of the four biopsy samples decreased to baseline values within 8.6 min. However, one current remained increased for longer than 11.6 min.
Out of 16 duodenal biopsy samples obtained from cats, eight responded to 200 µM serotonin (Table 2). No correlation between the level of ΔIsc and the time needed for maximum response was observed, while it took 45.0 sec to reach the highest amplitude of 32 µA/cm2, and the smallest increase (6 µA/cm2) was reached after 100.7 sec. The current only dropped back to the original baseline values in two cases (after 13.3 and 18.4 min, respectively). The short circuit current of two other biopsy samples remained at the maximum value for 18.5 min. The current of four biopsy samples steadily decreased with time, but did not reach baseline values, even after 16~18 min. In those cases, the experiment was continued with the application of the next substance after 16~18 min, regardless of the current values, to maintain viability of the tissue for the remainder of the experiments.
In total, only two biopsy samples responded to serotonin. The ΔIsc for both biopsy samples was 6 µA/cm2, and the times until the peak current was reached were 9.6 sec and 10.6 sec, respectively. Both of these biopsy samples also showed responses after exposure to histamine and phloridzin.
Fifteen duodenal biopsy samples obtained from cats and 51 obtained from dogs were exposed to 4 µM prostaglandin, but none showed any response.
Of 14 duodenal biopsy samples exposed to 5 µM forskolin, only one responded (Table 2).
Five of 51 biopsy samples tested responded to 5 µM forskolin with an increase in short circuit current (median ΔIsc of the 5 responding biopsy samples: 6 µA/cm2; range: 6~16 µA/cm2). One of these biopsy samples was from a dog in group 2, while four biopsy samples were from group 3. While the current of three biopsy samples slowly returned to baseline values after 6.1 ~11.4 min, two biopsy samples from one animal kept a stable current at maximum values for more than 4.2 min.
Out of 14 duodenal biopsy samples obtained from cats exposed to 40 mM glucose at the end of all experiments, only one responded (Table 2). Nine of 47 duodenal biopsy samples obtained from dogs responded to 40 mM glucose at the end of the experiment (Table 3). Three of those samples were from group 1, three from group 2, and three samples from group 3.
Fourteen duodenal biopsy samples obtained from cats were exposed to 600 µM ouabain. Eleven biopsy samples showed a reduction of current (Table 2).
Out of 44 biopsy samples obtained from dogs exposed to ouabain, 16 responded with a change in short circuit current. The median change in the short circuit current of the 16 biopsy samples that responded was 17 µA/cm2, ranging from 4 to 42 µA/cm2. However, the exposure to ouabain caused equivocal results: while the current of nine biopsy samples decreased, the Isc of seven biopsy samples increased.
In the present study, we evaluated the feasibility of an adapter-modified Ussing chamber for the investigation of endoscopically obtained duodenal biopsy samples from cats and dogs. Our results suggest that this modification of the conventional Ussing chamber method provides a feasible tool for future studies of intestinal transport in companion animals.
The mean (± SD) resistance of biopsy samples obtained from cats was 20.3 (± 10.8) Ω·cm2, while that of biopsy samples obtained from dogs was 17.3 (± 10.3) Ω·cm2. However, the resistance of stripped canine jejunum has previously been reported by Neirinckx et al. [24] (250.9 ± 109.2 Ω·cm2), and, while the standard deviation in these samples was about 50%, similar variations of tissue resistance were reported for the small intestine of rats (20 ~50 Ω·cm2) [27,29]. Since we transferred only the thickest and most intact looking biopsy samples into the Ussing chamber, it nearly always evaluated the full thickness biopsies. The presence of a few muscular cells could be nicely seen by a gentle curling of the biopsy. Since the individual animals were of varying weight and breed, their intestinal walls were of varying thickness; therefore, we cannot exclude an influence of biopsy thickness on absorptive and secretory capacity. Alterations in conductance values may also occur due to the physiologic loss of tissue integrity when tissues are maintained ex vivo, hypoosmosis, or stress of the host animal [23,32,33,34]. Stress may have contributed to the findings in this study, as most patients had significant gastrointestinal disease and had been hospitalized to perform a work-up.
Observed variations in the baseline current and the ΔIsc may be due to physiological variability of intestinal secretory/absorptive activity, but may also reflect the fact that we tested patients with a variety of gastrointestinal diseases [6]. The activation of current-generating transporters can be affected by the availability of intracellular ions, the cell volume, or modifications of membrane proteins [26]. Physical and psychological stress can also have a significant effect on epithelial transport and be associated with increased chloride secretion [23,34,32]. The gastrointestinal diseases of the patients enrolled in this study might also have caused alterations in response to some of the stimuli. For example, patients with small intestinal ulcerations have been reported to have an altered duodenal bicarbonate secretion [6]. Additionally, in inflamed tissue, Isc values may have been underestimated, as tissue conductance can be affected by a loss of tissue integrity. Chronically inflamed tissue is also known to contain down-regulated ion exchangers [30,36,37].
The animals investigated in the present study were affected by a variety of gastrointestinal diseases (Table 1). To correlate functional alterations of intestinal transport with morphological findings, canine duodenal samples were grouped into three categories. Interestingly, the total rate of responsive biopsies was highest (87.5%) for the biopsy samples from group 1, while 32.1% of the biopsies from group 3 responded to a given stimulus. These observations confirm the importance of tissue integrity when using the Ussing chamber method. Because most of the samples from group 3 showed lymphoplasmacytic inflammation, intact transport function of these biopsies would have been surprising. Thus, the results of the Ussing chamber experiments in this study can be correlated to histopathologic findings.
Despite a similar histopathologic diagnosis of biopsy samples in group 1 and group 2, the total rate of responsive biopsies, as well as absorptive and secretory capacity, was reduced in those in group 2. Obviously, not only morphologic diagnostic findings can be held responsible for the functional integrity of the gastrointestinal tract. The findings of this study support the observations that clinical signs of patients with gastrointestinal disease rarely correlate with histopathologic scores [8,9]. This discrepancy, which was reflected by our results once again, indicates the need for establishing techniques that investigate functional changes in the diseased gastrointestinal mucosa.
Subsequent application of several substances during the experiment may have led to unusual stress on the tissue specimens. Additionally, channels and transporters may already have been in a state of maximum conductance, resulting in an attenuated, or even absent response to further stimulation by cAMP. Therefore, our findings must be evaluated with caution.
Glucose-induced short circuit current has been suggested as a marker for villus integrity [21]. In our study, glucose failed to induce a change in Isc in biopsy samples from most dogs and cats. Not only did only a small number of samples respond to glucose at the end of the experiment, many biopsies did not react to this stimulus in the beginning of the experiment. Ferraris and Diamond [11] demonstrated that the gradient for phloridzin binding along the intestinal wall was parallel to the gradient for maximum glucose transport. Therefore, the normal positional gradient in glucose transport along the intestine arises from a gradient in transporter density, which in turn appears to be induced by the normal gradient in luminal glucose concentration. Therefore, the amplitude of ΔIsc reflects not only villus integrity, but also the overall absorptive capacity of the animal's small intestine [25].
A higher percentage of samples responded to phloridzin than to glucose for both canine (in all groups) and feline duodenal samples. The response to phloridzin demonstrated existing glucose uptake, although the sugar absorption could not reliably be demonstrated by adding 40 mM glucose. The most likely explanation for this observation is that the maximum glucose uptake occurred before 40 mM glucose was applied mucosally. As mentioned earlier, during set-up of the experiment, 10 mM glucose was applied to the serosal bathing solution, and 10 mM sorbitol was concurrently added to the mucosal buffer solution to maintain iso-osmolarity. While both sugars were dissolved in buffer solution during the equilibration period, they might have crossed the serosal/mucosal barrier due to leakage. The intestinal membranes of the diseased patients of this study may have had some leakage, and the tissue is not very tight; therefore, the 10 mM serosal glucose could have moved to mucosal buffer and been transported. To prevent this bias, we could have used fructose instead of glucose for enterocyte nutrition. Fructose is absorbed non-electrogenically; however, it is not known if fructose is metabolized as fast as glucose in the duodenum. Apart from a loss of tissue integrity, instrumental reasons for leakage might be a possibility. The diameter of the air suction adapter aperture might have been too wide, allowing the biopsy to be held in place, but enabling improper sealing at the edges. Additional studies investigating radio labeled fluxes are necessary to determine if this is the case. However, improper sealing of the biopsy to the adapter disc might be have caused the thin and slender feline duodenal biopsy samples. Indeed, only two out of 12 phloridzinpositive samples also exhibited a response to glucose, whereas, among the thicker duodenal biopsies obtained from dogs, nearly all phloridzin-positive samples also responded to glucose (21 of 25 samples). The intestinal walls of cats are thinner than those of dogs. Hence, the air suction space might have been appropriate for biopsy samples obtained from dogs, but too wide for mounting the intestinal samples from cats. Thus, we believe that the vacuum space of the adapter-discs should be smaller than 50 µm when they are used for companion animals.
The overall response to secretory compounds of feline and canine biopsy samples was lower (50~0%) than the response to absorptive stimuli (70.6~11.8%). The highest median ΔIsc after exposure to a secretory compound was observed after serotonin exposure for duodenal biopsy samples obtained from cats (19 µA/cm2), and after histamine exposure for duodenal biopsy samples obtained from dogs (8 µA/cm2). In both species, those responses were lower than the median ΔIsc after exposure to absorptive stimuli (biopsy samples obtained from cats: 28 µA/cm2, duodenal biopsy samples obtained from dogs: 34 µA/cm2). While the small intestine is known for its large capacity to absorb nutrients, these findings are not surprising considering the state of inflammation of the majority of tissues. The investigation of a diseased study population might have been responsible for a reduced secretory response. For example, histamine is responsible for inhibition of prostaglandin E2 stimulated bicarbonate secretion [16]. Increased histamine concentrations in the gastrointestinal tract, as can be found with a variety of gastrointestinal diseases, might have resulted in a lower short circuit current baseline value and possibly a lower magnitude of ΔIsc. This might be supported by the fact that the response of duodenal biopsy samples to histamine was not only most frequent in biopsy samples of canine group 1 (group 1: 25%, group 2: 0%, group 3: 10.7%), but also showed the highest median ΔIsc (13 µA/cm2). Additionally, the application of histamine before the application of prostaglandin might have inhibited the response to prostaglandin. This phenomenon has previously been demonstrated in rabbits in which the response to prostaglandin was significantly reduced when the tissue was pretreated with histamine when compared to the response to prostaglandin alone [16]. The occurrence of the same mechanisms in companion animals might explain why none of the duodenal biopsy samples in either cats or dogs responded to prostaglandin.
The limitation of working with a diseased study population might also have affected the response to serotonin. Donowitz et al. [10] demonstrated an altered serotonin response based on the severity of histological abnormalities of rabbit intestine. Moreover, while 50% of feline duodenal biopsy samples responded to the application of serotonin, only two of 51 tested canine duodenal biopsy samples changed their short circuit current. Obviously, species-specific investigations of intestinal sensitivity to various chemical compounds and the expression of various drug receptors need further research. As mentioned above, only limited conclusions can be drawn regarding the response to each substance as the magnitude of Isc changes might have been altered due to exposure of chemicals applied earlier during the experiment. However, logistical reasons prevented investigation of each biopsy for responses to a single substance alone. Based on this study, dose-responsive studies with the stimuli tested can now be performed to validate tissue response.
The effect of 600 µM ouabain on duodenal biopsy samples in our study caused equivocal results. While the short circuit current of most samples decreased, the current of some samples increased after ouabain exposure. Once again, the observed changes could not be associated with a certain group of patients or type or severity of histopathological changes. While inflammation decreases the activity of Na-K-ATPase via both decreased expression on basolateral membrane and potential modifications, the variable responses are likely due to tissue inflammation and integrity. Multiple tissue responses to individual ouabain doses can be investigated in the future to characterize the viability of the tissue response.
Another interesting finding was the time period until maximum current values were reached. The time period until reaching a maximum response to ouabain was 17.2 min, while responses to all other substances occurred within seconds. The long lasting effect of ouabain is not unusual and is in agreement with other studies [27,29]. The disadvantage of such a long observation time, especially when only minor changes of ΔIsc can be observed, is that it results in some uncertainty regarding whether those changes are due to the influence of ouabain or reflect deterioration of tissue viability. The action of ouabain is due to an irreversible inhibition of the sodium potassium pump (Na+/K+ ATPase) [26,28]. The Na+/K+ ATPase, located on the basolateral membrane of the enterocyte, is another target for indirectly enabling chloride secretion. However, the equivocal responses of our tested biopsy samples to ouabain suggest that the entry mechanism for chloride across the basolateral membrane is probably not entirely due to the activity of this cotransporter. It is well known that chloride entry at the basolateral side of the enterocyte is dependent on active sodium potassium chloride transporter 1 (NKCC-1), and the activity of that transporter is largely regulated by sodium gradients. Therefore, the decrease in the sodium gradient of the enterocyte in response to ouabain would be a secondary mechanism decreasing basolateral Cl entry. The equivocal effect of ouabain on the short circuit current has also been observed in previous studies and shown to be correlated with the preparation of tissue sample [29]. If the mucosa was still attached to the plexus submucosus, an increase of Isc could be observed, while the effect of ouabain on isolated mucosa resulted in a decrease of current, followed by a further decline of Isc [29].
As mentioned above, a major limitation of this investigation was the non-homogeneous study population, as it included dogs and cats of different breeds and dogs of different disease states. Some breeds are known to be pre-disposed to gastrointestinal disorders, such as Siamese cats, Soft Coated Wheaten Terriers, Boxers, and German Shepherds [15,18,22]. This study included one Siamese cat, one Soft Coated Wheaten Terrier, two German Shepherds, and one Boxer. All except two healthy dogs underwent diagnostic endoscopy for the routine work-up of their suspected gastrointestinal disease. Many of these animals had severe disease and were treated aggressively, even before the biopsy samples had been collected. Diagnosis based on a complete clinical work up, including clinical history, clinical examination, blood chemistry, complete blood count, histopathologic evaluation of intestinal biopsies, and/or response to treatment, identified several different diseases, including IBD, protein-losing enteropathy, and intestinal adenoma. Each of these disorders may have affected intestinal transport physiology in a different fashion.
Moreover, diseased animals enrolled in our study received specific medications during and/or prior to hospitalization, which might also have affected mucosal transport physiology. It is known that glucocorticoids and mineralocorticoids upregulate expression of the epithelial sodium channel (ENaC) and Na+/K+ ATPase, and induce electroneutral sodium absorption, whereas high doses also increase electrogenic sodium absorption [2,3,5]. Increases of sodium transport can also be induced by Polyene antibiotics (e.g., amphotericin B, nystatin) or other substances that decrease tissue resistance [13].
The non-homogeneous study population included animals of varying ages (7 to 149 months). The effect of age on ion transport has been studied in several species, and cAMP-dependent chloride secretion was reported to be lower in animals of higher age [12,14,20,38]. An impact of the collection procedure on tissue resistance was previously reported by Stevens, and may also have played a role in our experiments [35]. In this study, mechanical damage to the epithelium might have occurred during biopsy collection. Fragile duodenal villi can easily be damaged by endoscopic biopsy forceps. Endoscopy and biopsy collection was performed by several clinicians with varying degrees of experience. Damaged tissue may exhibit a higher conductance compared to good quality biopsies, and trauma-induced hormonal stimulation of the samples cannot be excluded. Despite this disadvantage, several studies showed the feasibility of evaluating endoscopically-collected biopsy samples with Ussing chambers [4,6,7,21]. Understanding the dysfunction of intestinal epithelial transport functions, such as transporter up- or downregulation in companion animals affected by chronic enteropathies, might allow further characterization of these diseases based on the electrophysiologic parameters, and might improve our understanding of those diseases or even allow the development of new treatments for these diseases. Overall, this study provides initial evidence of the ability to use an adapter-modified Ussing chamber for the evaluation of transport physiology in endoscopically-collected duodenal biopsy samples obtained from cats and dogs. The adapter modified Ussing chamber can be used to investigate the action of specific substances on intestinal ion transport and thus allows the investigation of intestinal diseases where transporter function might be impaired. The advantage of evaluating endoscopic biopsy samples, rather than surgically obtained tissue samples, cannot be overemphasized as it enables investigation of larger study populations and longitudinal studies.
Figures and Tables
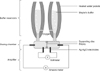 | Fig. 1Sketch of the adapter modified Ussing chamber. The principal measurements of the Ussing chamber are based on two functional units: the physiological system, and the electrical circuit. The buffer reservoirs of 10 mL each of gassed and heated buffer enabled short-term physiological transport function of the intestinal mucosa. The amplifier was used to perform the electrical measurements. |
References
1. Allenspach K, Wieland B, Gröne A, Gaschen F. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med. 2007; 21:700–708.

2. Bastl CP. Regulation of cation transport by low doses of glucocorticoids in in vivo adrenalectomized rat colon. J Clin Invest. 1987; 80:348–356.

3. Batt RM, Scott J. Response of the small intestinal mucosa to oral glucocorticoids. Scand J Gastroenterol Suppl. 1982; 74:75–88.
4. Bijlsma PB, Backhaus B, Weidenhiller M, Donhauser N, Hahn EG, Raithel M. Food allergy diagnosis by detection of antigen-induced electrophysiological changes and histamine release in human intestinal biopsies during mucosa-oxygenation. Inflamm Res. 2004; 53:Suppl 1. S29–S30.

5. Binder HJ, Turnamian SG. Differential effects of corticosteroids on active electrolyte transport in the mammalian distal colon. Acta Vet Scand Suppl. 1989; 86:174–180.
6. Bukhave K, Rask-Madsen J, Hogan DL, Koss MA, Isenberg JI. Proximal duodenal prostaglandin E2 release and mucosal bicarbonate secretion are altered in patients with duodenal ulcer. Gastroenterology. 1990; 99:951–955.

7. Cohen-Cymberknoh M, Yaakov Y, Shoseyov D, Shteyer E, Schachar E, Rivlin J, Bentur L, Picard E, Aviram M, Israeli E, Kerem E, Wilschanski M. Evaluation of the intestinal current measurement method as a diagnostic test for cystic fibrosis. Pediatr Pulmonol. 2013; 48:229–235.

8. Craven M, Simpson JW, Ridyard AE, Chandler ML. Canine inflammatory bowel disease: retrospectice analysis of diagnosis and outcome in 80 cases (1995-2002). J Small Anim Pract. 2004; 45:336–342.

9. Day MJ, Bilzer T, Mansell J, Wilcock B, Hall EJ, Jergens A, Minami T, Willard M, Washabau R. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: a report from the World Small Animal Veterinary Association Gastrointestinal Standardization group. J Comp Pathol. 2008; 138:Suppl 1. S1–S43.

10. Donowitz M, Charney AN, Heffernan JM. Effect of serotonin treatment on intestinal transport in the rabbit. Am J Physiol. 1977; 232:E85–E94.

11. Ferraris RP, Diamond JM. Use of phlorizin binding to demonstrate induction of intestinal glucose transporters. J Membr Biol. 1986; 94:77–82.

12. Freeman HJ, Quamme GA. Age-related changes in sodium-dependent glucose transport in rat small intestine. Am J Physiol. 1986; 251:G208–G217.

13. Frizzell RA, Turnheim K. Ion transport by rabbit colon: II. Unidirectional sodium influx and the effects of amphotericin B and amiloride. J Membr Biol. 1978; 40:193–211.

14. Greig ER, Mathialahan T, Boot-Handford RP, Sandle GI. Molecular and functional studies of electrogenic Na+ transport in the distal colon and rectum of young and elderly subjects. Gut. 2003; 52:1607–1615.

15. Hall EJ, Rutgers HC, Scholes SFE, Middleton DJ, Tennant BJ, King NM, Kelley DF. Histiocytic ulcerative colitis in boxer dogs in the UK. J Small Anim Pract. 1994; 35:509–515.

16. Hogan DL, Yao B, Barrett KE, Isenberg JI. Histamine inhibits prostaglandin E2-stimulated rabbit duodenal bicarbonate secretion via H2 receptors and enteric nerves. Gastroenterology. 1995; 108:1676–1682.

17. Jergens AE, Crandell JM, Evans R, Ackermann M, Miles KG, Wang C. A clinical index for disease activity in cats with chronic enteropathy. J Vet Intern Med. 2010; 24:1027–1033.

18. Jergens AE, Moore FM, Haynes JS, Miles KG. Idiopathic inflammatory bowel disease in dogs and cats: 84 cases (1987-1990). J Am Vet Med Assoc. 1992; 201:1603–1608.
19. Jergens AE, Schreiner CA, Frank DE, Niyo Y, Ahrens FE, Eckersall PD, Benson TJ, Evans R. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med. 2003; 17:291–297.

20. Kosik-Bogacka D, Tyrakowski T. Ion transport in rabbit caecum at 12 and 36 months of age. J Physiol Pharmacol. 2004; 55:Suppl 2. 117–128.
21. Larsen R, Mertz-Nielsen A, Hansen MB, Poulsen SS, Bindslev N. Novel modified Ussing chamber for the study of absorption and secretion in human endoscopic biopsies. Acta Physiol Scand. 2001; 173:213–222.

22. Littman MP, Dambach DM, Vaden SL, Giger U. Familial protein-losing enteropathy and protein-losing nephropathy in Soft Coated Wheaten Terriers: 222 cases (1983-1997). J Vet Intern Med. 2000; 14:68–80.

23. Meddings JB, Swain MG. Environmental stress-induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology. 2000; 119:1019–1028.

24. Neirinckx E, Vervaet C, Michiels J, De Smet S, Van den Broeck W, Remon JP, De Backer P, Croubels S. Feasibility of the Ussing chamber technique for the determination of in vitro jejunal permeability of passively absorbed compounds in different animal species. J Vet Pharmacol Ther. 2011; 34:290–297.

25. Polentarutti BI, Peterson AL, Sjöberg AK, Anderberg EK, Utter LM, Ungell AL. Evaluation of viability of excised rat intestinal segments in the Ussing chamber: investigation of morphology, electrical parameters, and permeability characteristics. Pharm Res. 1999; 16:446–454.
27. Pratha VS, Thompson SM, Hogan DL, Paulus P, Dreilinger A, Barrett KE, Isenberg JI. Utility of endoscopic biopsy samples to quantitate human duodenal ion transport. J Lab Clin Med. 1998; 132:512–518.

28. Pressley TA. Structure and function of the Na, K pump: ten years of molecular biology. Miner Electrolyte Metab. 1996; 22:264–271.
29. Rangachari PK, McWade D. Epithelial and mucosal preparations of canine proximal colon in Ussing chambers: comparison of responses. Life Sci. 1986; 38:1641–1652.

30. Rocha F, Musch MW, Lishanskiy L, Bookstein C, Sugi K, Xie Y, Chang EB. IFN-γ downregulates expression of Na+/H+ exchangers NHE2 and NHE3 in rat intestine and human Caco-2/bbe cells. Am J Physiol Cell Physiol. 2001; 280:C1224–C1232.
31. Ruhnke I, DeBiasio JV, Suchodolski JS, Newman SJ, Musch MW, Steiner JM. Adapter-modified Ussing chamber enables evaluation of endoscopically-obtained biopsy samples from cats and dogs. Res Vet Sci. 2012; 93:1454–1461.

32. Saunders PR, Kosecka U, McKay DM, Perdue MH. Acute stressors stimulate ion secretion and increase epithelial permeability in rat intestine. Am J Physiol. 1994; 267:G794–G799.

33. Söderholm JD, Hedman L, Arthursson P, Franzén L, Larsson J, Pantzar N, Permert J, Olaison G. Integrity and metabolism of human ileal mucosa in vitro in the Ussing chamber. Acta Physiol Scand. 1998; 162:47–56.
34. Söderholm JD, Perdue MH. Stress and gastrointestinal tract. II. Stress and intestinal barrier function. Am J Physiol Gastrointest Liver Physiol. 2001; 280:G7–G13.
35. Stevens CE. Transport of sodium and chloride by the isolated rumen epithelium. Am J Physiol. 1964; 206:1099–1105.

36. Sugi K, Musch MW, Field M, Chang EB. Inhibition of Na+, K+-ATPase by interferon γ down-regulates intestinal epithelial transport and barrier function. Gastroenterology. 2001; 120:1393–1403.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download