Introduction
Diabetes mellitus is a common metabolic disorder in canines. Affected subjects are usually more than 5 years of age, and there is a predisposition in females [9]. In dogs, insulin resistance (IR) is suspected when hyperglycemia is still detected despite administration of insulin doses greater than 1.0 to 1.5 IU/kg [5]. Definitions of resistance to exogenously administered insulin vary, but all are based on the dose insulin administered and the resultant blood glucose concentrations [6]. IR is caused by an increase in circulating counter regulatory hormones (glucagon, glucocorticoids, catecholamines and growth hormone). These stress-related diabetogenic hormones increase as a result of concurrent diseases, endocrine disorders or exogenous administration. Progesterone (P4) also induces IR by stimulating growth hormone (GH) production in mammary glands [4,6]. Increases in circulating P4 concentration are associated with exogenous administration and physiologic conditions such as diestrus and pregnancy. In pregnant and non-pregnant bitches, increases in GH are related to increases in circulating P4 and appears to be secondary to synthesis of GH in mammary glands. It has been clearly demonstrated that P4 can stimulate mammary GH hypersecretion during the non-pregnant luteal phase [6]. Elevated GH plasma concentrations are characterized by the absence of a pulse pattern and insensitivity to stimulation and inhibition tests, with the exception of inhibition by the progesterone antagonist RU486. In hypophysectomized dogs, no absence or decrease in plasma GH concentrations was detected, which suggested the presence of an extra-pituitary origin. Selman et al. [16] demonstrated that mammary glands were effectively the source of GH concentrations. In human and felines, increased GH concentrations have been observed after progestin treatment [8]. Aglepristone, which is the first P4 receptor blocker licensed for veterinary use, has been successfully used for pregnancy interruption, pyometra medical therapy and treatment of feline fibroadenomatous mammary hyperplasia [2,3]. Aglepristone binds to uterine P4 receptors with affinity three times greater than P4 itself in bitches and nine times greater in queens. Aglepristone blocks receptors, but also displaces P4 already bound to receptors [11]. Aglepristone does not modify plasma concentrations of P4, but indirectly induces uterine contractions and cervix dilatation after multiple injections [11]. Polisca et al. [13] demonstrated that aglepristone in dogs reduces the length of P4 secretion by CL and accelerates the luteolytic process, and that this effect persists for about 6 to 8 days. Several side effects have been observed after administration of aglepristone, such as anorexia, restlessness, depression, vomiting, diarrhea decrease in body temperature and local inflammatory reaction after injection [11]. The present study was conducted to investigate the use of aglepristone for the treatment of insulin-resistant diabetes mellitus during the luteal phase.
Materials and Methods
Animals
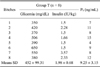
All bitches included in the study were diabetic subjects under insulin therapy that were referred to our Veterinary Teaching Hospital over a period of two years for insulin-resistant diabetes mellitus that developed during mid-luteal phase of the estrous cycle (25th~30th day of diestrus). The luteal phase was established by vaginal cytology examined upon hematoxylin-eosin staining and blood P4 concentrations. Criteria for selection of patients were: glycemia persistently > 200 mg/dL over a 12 h period, despite treatment with more than 1.5 IU/Kg BID of insulin; clinical and laboratory findings (P4 > 2 ng/mL) indicative of diestrus. Diet, training, environmental factors and the insulin protocol administration were similar for all subjects. Exclusion criteria were the presence of other disorders or treatments that can cause insulin resistance. Eight intact bitches of different breeds and ages (9 to 15 years; mean ± standard deviation [SD]: 10.9 ± 1.46 years) met the inclusion criteria, as treatment group (group T; n = 8) and were therefore classified as having a P4 induced insulin-resistant diabetes mellitus (Table 1). The control group (group C; n = 6) was composed by six diabetic bitches of mixed breeds and ages (8 to 12; mean ± SD: 10 ± 1.41) under insulin therapy in the same phase of the estrous cycle (25th~30th day of diestrus, Table 2). The study was carried out in accordance with the Italian Legislation on Animal Care (DL 116/92).
Blood sample collection
Blood samples (4 mL) for the determination of P4 concentration (a commercial available Radioimmunoassay-RIA for canine) and GH (commercially available RIA for canine and porcine GH; Linco Research, USA) were collected before and after treatment with aglepristone by cephalic venipuncture and immediately transfer to ice-chilled EDTA-coated tubes for GH determination, as well as to tubes without anticoagulant for P4 determination. All samples were centrifuged at 4℃ for 10 min, after which plasma was stored at -25℃ until assayed. Blood samples for determination of the pulsatile plasma profile of GH were collected at 15 min intervals between 8:00 AM and 14:00 PM before aglepristone administration and five days after the last aglepristone treatment [2]. Serial blood glucose curves were performed on day 0, 5, 12 and 20 after the beginning of treatment.
Therapy
All subjects were treated with porcine insulin zinc suspension (Caninsulin; Intervet, Italy) as reported in Table 1. Bitches in group T were treated with 10 mg/kg subcutaneously (SC) aglepristone (Alizin; Virbac, Italy) on day 1, 2, 9 and 17 in diestrus bitches. The control group received porcine insulin zinc suspension ≤1 IU/Kg (Caninsulin; Intervet) as well as saline solution (0.3 mL/kg, SC) on days 1, 2, 9, 17.
Results
Prior to aglepristone administration, the mean serum P4 concentrations averaged 9.25 ± 3.15 and 10.50 ± 2.06 ng/mL in group T and group C, respectively (p > 0.05) (Tables 1 and 2), while the mean plasma GH concentrations were 2.37 ± 0.17 µg/L in group T and 1.80 ± 0.11 µg/L in group C (p < 0.05). Following treatment, the plasma GH concentration in group T was significantly lower 1.7 ± 0.6 µg/L (Tables 3 and 4); however, in group group C it was 1.68 ± 0.07 µg/L, which was not a significant difference (Table 4). At the end of treatment, the mean P4 serum concentrations were 8.37 ± 2.24 and 9.33 ± 1.79 ng/mL in group T and group C, respectively (Tables 5 and 6). The final P4 values of group T and C did not differ significantly. In IR subjects (group T), no significant variations in glycemia mean values were observed between day 0 and day 5 (p < 0.05). At day 12 and 20, the mean concentration of blood glucose was significantly lower (p < 0.05) than on day 0 (Table 5). In the control group (Table 6), between the animals, blood glucose means concentrations were not significant (p > 0.05). On day 20, diabetes mellitus was well controlled in all the bitches (group T; Table 5). This results in group T allowed us to keep insulin dosage less than 1 IU/kg during the following 6 weeks, while before the aglepristone treatment the mean insulin dosage was 1.98 ± 0.68 IU/Kg (Tables 1 and 5). No side effects were recorded during aglepristone administration. The bitches were spayed within two months because they were euglycemic. Six months later they were still euglycemic.
Discussion
Glucose is the main source of energy for all body tissue, except cardiac and skeletal muscle. Blood glucose concentration is a reflection of gastrointestinal absorption, glycogenolysis, gluconeogenesis and glucose consumption by tissues. Glucose production and its metabolism occur as a result of interaction of hormones, cytokines and intracellular transport [10]. Several glucose transporters have been identified, such as GLUT-4 in skeletal muscle, cardiac and adipose cells, as well as other glucose transporters (GLUT-1; GLUT-2) independent from insulin action in the brain, liver, kidney, placenta, sperm, adipocytes and erythrocytes. Increasing blood glucose concentrations stimulate insulin secretion, while low blood glucose concentrations suppress insulin secretion and stimulate production of various hormones (glucagon, epinephrine, norepinephrine, growth hormone, cortisol). There is growing evidence suggesting that GH modulates insulin sensitivity via multiple mechanisms due to the influence of crosstalk between GH/Insulin-like growth factor-1 (IGF-1) and insulin signaling, including reduced tyrosine kinase (TK) activity. However, insulin resistance alone appears likely to cause diabetes, once only few bitches in diestrus develop canine diabetes mellitus, which is undoubtedly a multifactorial disease, because there is growing evidence suggesting that GH modulates insulin sensitivity by multiple mechanisms, due to the influence of crosstalk between GH/insulin-like growth factor1 (IGF-1) [14]. Pregnancy is associated with IR in humans and dogs, which occurs in response to suppression of the intracellular transport of glucose and its increasing concentration in blood. P4, estradiol, growth hormone, placental lactogen and placental cytokines all play important roles in causing insulin resistance [10,17]. There are several causes of resistance to exogenous insulin that do not lead to IR, such as improper handling and administration of insulin. Somogyi effect, which occurs when pronounced hyperglycemia develops in response to severe insulin-induced hypoglycemia, may also cause misdiagnosis of insulin resistance. In diabetic patients, sudden severe insulin-induced hypoglycemia results in development of protective mechanisms involving secretion of catecholamines, glucocorticoids, glucagon, and GH, which lead to pronounced hyperglycemia [6]. Actually, in subjects showing Somogyi effect, the activity of insulin is high. In our cases, elevated doses of insulin did not induced fatal hypoglycemia. Gestational diabetes mellitus is a clinical condition characterized by a variable degree of glucose intolerance that can develop during pregnancy, parturition and diestrus [7,10]. During pregnancy, this disease is a rare condition [1]. Moreover the basis of this mechanism has not been fully elucidated. It is likely that gestational diabetes mellitus exerts a negative impact on fetuses and can compromise pregnancy and newborn viability [1,10]. A high risk of adverse fetal events, including abortion, small unthrifty pups and overly large pups (macrosomia), has been described in diabetics bitches. Vascular effects of diabetes may reduce placental blood supply, contributing to abortion or poorly-grown pups [12]. Pregnant bitches show greater insulin resistance than non-pregnant diestrous bitches. During the luteal phase basal GH secretion and P4 concentrations are high. The canine mammary gland expresses genes encoding GH, and its expression is strongly stimulated by P4. The long exposure to high circulating levels of P4 during the luteal phase may even result in excess GH with acromegaly and/or diabetes mellitus in bitches [7]. Previous studies identified foci of hyperplastic ductular epithelium of the mammary gland as the site of origin of GH excess induced by progestins. These observations are consistent with the central role of progestins in GH gene expression in canine mammary gland and allow for a target endocrine therapy with progesterone receptor blockers with progestin-induced mammary-derived GH hypersecretion [2]. Some authors have also found that administration of antiprogestin resulted in decreased plasma GH concentrations and normalization of plasma IGF-I concentrations in bitches with progestin-induced acromegaly [2]. The insulin-antagonist action of GH (progestin-induced hypersecretion of GH) may result in hyperglycemia and diabetes mellitus. Hyperglycemia can be reversed after correction of high GH levels [2]. In our cases, the P4 levels in the luteal phase resulted in hyperglycemia due to IR [14]. Additionally, treatment with aglepristone (RU 46534) was useful to control the hyperglycemia levels in diestrus bitches, which was clearly demonstrated by the P4 levels (> 2 ng/mL) at the end of the aglepristone treatment. Our findings are in agreement with those of Watson et al. [18], who found that administration of the mifepristone resulted in a decreased GH plasma concentration in bitches with progestin-induced acromegaly. Polisca et al. [13] showed lower progesterone levels after aglepristone administration, which was likely related to acceleration of the luteolytic process. The gradual decline of progesterone observed in treated dogs suggested that antiprostagen triggers an anticipated, physiological-like luteolytic process [13]. In our opinion, the results of this prospective study suggest that aglepristone represents an effective therapeutic choice. Nevertheless, the best and definitive treatment for insulin resistance due to progesterone is gonadectomy as soon as possible [15], while the use of aglepristone should be reserved only for cases in which surgery is not possible or authorized by the owners. Ovariohysterectomy promotes a quick drop in P4 levels, which is of great interest to managing diabetic bitches. In conclusion, the results of the present study demonstrated that aglepristone treatment significantly decreased blood glucose concentration in bitches with progestin-induced insulin-resistance.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download