Abstract
The interferon-gamma (IFN-γ) assay is employed as a complementary diagnostic test for bovine tuberculosis (BTB) in many countries. To simplify this assay, we established a 96-well plate format using the ESAT-6 and CFP-10 antigens and then employed it to determine the extent of Mycobacterium (M.) bovis infection in dairy herds with a history of BTB outbreaks in a country where only selective culling is practiced. The sensitivity and specificity of this IFN-γ assay were 85.9% and 100%, respectively, based on comparison with the conventional single intradermal tuberculin test (SIDT). The IFN-γ assay was also positive in 30.4% and 36.8% of SIDT-negative animals from herds with recent and remote BTB outbreaks, respectively. Of 14 SIDT-negative, IFN-γ positive cattle, five (35.7%) were culture positive and an additional six were positive based on a polymerase chain reaction-based test for M. bovis. Therefore, the IFN-γ assay has the potential to serve as a specific and sensitive test for M. bovis infection in dairy cattle. Further, the results indicated that a substantial portion of SIDT-negative animals in herds with previous BTB outbreaks were actually infected with M. bovis. Accordingly, the present selective-culling strategy may require modifications to include this more sensitive assay.
Bovine tuberculosis (BTB) is one of the major diseases caused by Mycobacterium (M.) bovis, a member of the Mycobacterium tuberculosis complex. M. bovis can infect cattle, deer, wildlife, and humans via respiratory routes [8]. BTB has been spreading worldwide and is a major issue concerning human public health, particularly in areas where unpasteurized milk is consumed. BTB also threatens the agricultural economies of many countries [10]. A testing and culling strategy has been widely adopted to eradicate BTB from dairy cattle herds. The diagnosis of BTB is primarily based on the single intradermal test (SIDT), which measures M. bovis-specific cell mediated immune (CMI) responses following intradermal injection of bovine purified protein derivative (PPD) [9]. The use of SIDT and the culling of M. bovis-infected animals resulted in a rapid decrease in the incidence of BTB. However, as the prevalence of M. bovis infection in cattle decreased, the sensitivity and specificity of SIDT were reduced due to the rarity of infected animals and infection by non-tuberculous mycobacteria (NTM), respectively [6]. Therefore, a more sensitive and specific diagnostic test is needed. Recently, an interferon-gamma (IFN-γ) assay, the Bovigam Bovine Gamma Interferon Test (Prionics, Switzerland), was reported to detect M. bovis-infected animals with a sensitivity of 82 ~ 100% and a specificity of 94 ~ 100% [3,22].
Although culling of M. bovis-infected animals is recommended for effective eradication of BTB, cost constraints have resulted in only SIDT-positive animals actually being culled in the national BTB control programs of many countries, including South Korea. This policy has the potential to leave M. bovis-infected but SIDT-negative animals in herds that have had BTB outbreaks [3]. Repeated SIDT of all animals in herds with BTB outbreaks followed by selective culling may eventually be successful in eradicating BTB, but its cost will be much higher than a culling strategy that is initially effective. It would also be more cost effective if a selective culling practice was applied based on a highly sensitive assay for the detection of M. bovis infection.
With recent development of the IFN-γ assay, it is now of great interest to determine the extent of M. bovis infection in cattle herds from which all SIDT-positive animals have been culled. Bovine IFN-γ assays generally utilize bovine PPD and avian PPD antigens to stimulate peripheral lymphocytes to eliminate non-specific responders due to exposure to NTM [16,20,21]. However, since M. tuberculosis complex-specific antigens such as early secreted antigenic target protein 6 kDa (ESAT-6) and culture filtrate protein 10 kDa (CFP-10) have been widely applied for detection of latent tuberculosis infection in humans, it would be more applicable for diagnostic laboratories to use these antigens instead of bovine and avian PPDs. Therefore, this study was conducted to establish an IFN-γ assay using the ESAT-6 and CFP-10 antigens and to determine the extent of M. bovis infection in cattle herds in which only SIDT-positive animals have been culled. Using this assay, we found that more than 30% of SIDT-negative cattle in herds with only partial culling were IFN-γ-positive, indicating that the IFN-γ assay may be more effective than SIDT at detecting M. bovis infection.
Animals used in this study were Holstein Friesian cattle aged over 1 year from (i) four dairy farms that had had no SIDT-positive cattle for more than 5 years based on repeated annual testing (n = 100), (ii) four dairy farms that had a history of BTB, but did not have any SIDT-positive animals within the current testing in a year (remote outbreak) (n = 114), and (iii) 11 farms that had at least one SIDT-positive animal within the current testing in a year (recent outbreak) (n = 260). All dairy farms were located in Northern Gyoenggi Province, Korea. SIDT-positive cattle were used as positive controls (n = 135), while animals from BTB-free farms were used as a negative control (n = 100).
Cattle were injected with 100 µL of bovine PPD (2 mg/mL) into the caudal fold, and the results of this test were based on the skin thickness determined 48 ~ 72 h after injection. The animals were considered positive if there was an increase of 5 mm or more in skin thickness, borderline-positive if the increase in skin thickness was more than 3 mm but less than 5 mm, and negative if the skin thickened by no more than 3 mm.
Heparinized blood samples were collected from each animal and delivered to the laboratory within 8 ~ 10 h of blood collection. Whole blood cultures were performed in 96-well plates in aliquots of 200 µL/well. Each aliquot was stimulated with pokeweed mitogen (PWM; Sigma-Aldrich, UK), a mixture of recombinant ESAT-6 and CFP-10 antigens, which were expressed in Escherichia coli as previously described [4,19], and phosphate buffered saline (PBS). PWM and PBS were used as positive and negative controls, respectively. The final concentration was 10 µg/mL for the antigen cocktail (5 µg/mL each of ESAT-6 and CFP-10) and 5 µg/mL for PWM. Supernatants were harvested after incubating the plates at 37℃ in a humidified 5% CO2 incubator for 18 ~ 24 h. IFN-γ was then determined by a sandwich enzyme-linked immunosorbent assay (ELISA). Briefly, the wells were coated overnight at 4℃ with 100 µL of 1 µg/mL anti-bovine IFN-γ antibody (AbD Serotec, UK) in 50 mM carbonate buffer (pH 9.5). After blocking the wells with 10% fetal calf serum (FBS) in PBS containing 0.05% Tween (PBS-T) (assay diluent), culture supernatants were added to the wells and the samples were incubated at 4℃ overnight. After washing the plates, 100 µL of 1 µg/mL biotin-conjugated anti-bovine IFN-γ antibody (AbD Serotec) in assay diluent were added and the samples were incubated for 60 min. After further washing, 100 µL of streptavidin-horseradish peroxidase (HRP; AbD Serotec) diluted 1 : 10,000 in assay diluent were added and incubated for 30 min. After the final wash, tetramethylbenzidine (KPL, USA) was added to the wells. The reaction was stopped after 25 min by the addition of 50 µL of 2.5N H2SO4, at which time the absorbance at 450 nm was read. Recombinant bovine IFN-γ (AbD Serotec) was used to generate a standard curve and IFN-γ levels were reported as picograms of protein per milliliter of supernatant. Prior to analysis, the mean absorbance value from medium control wells was subtracted from that of antigen-stimulation wells. Blood culture with antigens and the IFN-γ ELISA were both run in duplicate.
Hilar lymph nodes were homogenized and treated with 2% NaOH for 15 min, then centrifuged at 3,080 × g for 15 min. Next, the supernatant was discarded, and tissue homogenates were re-suspended in PBS. The centrifugation step was then repeated and the supernatant was discarded, after which the residues were inoculated onto slopes of Ogawa medium containing 0.05% pyruvate and incubated for 12 weeks at 37℃. For DNA extraction, lymph node homogenates were prepared using a DNeasy Blood and Tissue kit (Qiagen, Germany) according to the manufacturers' instructions.
Smart Taq Pre-Mix (Solgent, Korea) was used for polymerase chain reaction (PCR) amplification, together with DNA prepared as described above and primers specific for a 113 bp IS1081 amplicon (5'-CTGCTCTCGACGTTCATCGCCG-3' and 5'-TGGCGGTAGCCGTTGCGC-3') [18]. The PCR cycle consisted of an initial denaturation step of 95℃ for 7 min, followed by 35 cycles of 30 sec at 94℃, 60 sec at 58℃, and 30 sec at 72℃, and then a final extension step of 5 min at 72℃. The PCR products were subsequently analyzed by electrophoresis with using 1.5% agarose gels (Bioneer, Korea) in 1× Tris-acetic acid-EDTA buffer (pH 7.2). A 100-bp DNA ladder (Bioneer) was used to estimate the size of the PCR products.
Data were analyzed using GraphPad Prism 5 (GraphPad Software, USA). A receiver operating characteristic (ROC) curve was generated for the ESAT-6 and CFP-10 antigen cocktail to select the cutoff values that most effectively discriminated positive from negative samples. A student's t test was used to compare the mean IFN-γ levels, and the Mann-Whitney U test was used to compare nonparametric unpaired data. A p value < 0.05 was considered to be significant.
A whole blood assay was established in a 96-well culture plate format to determine IFN-γ production after stimulation with the recombinant ESAT-6 and CFP-10 antigen cocktail. The IFN-γ concentration in each well was determined by comparing the OD values to those of wells containing recombinant IFN-γ. Standard curves were derived from 8 concentrations ranging from 0.078 ng/mL to 10 ng/mL (Fig. 1), and each plate included its own standard curve. As the samples were diluted 1 : 2 before the assay, the maximum detectable concentration was 20 ng/mL. The IFN-γ concentrations of SIDT-positive cattle were then compared with those of SIDT-negative cattle. The majority of the 135 SIDT-positive cattle produced more than 0.5 ng/mL of IFN-γ, while none of the 100 SIDT-negative cattle from BTB-free herds produced this level of IFN-γ (Fig. 2). To account for the influence of other intrinsic factors that could affect the IFN-γ response, interpretation criteria of the results were established based on the IFN-γ assay detection limit and the results in SIDT-negative control animals and the criteria of the IFN-γ assay for diagnosis of M. tuberculosis infection in humans (QuantiFERON-TB; Cellestis, Australia). In this study, animals that gave an apparently negative result and produced less than 0.16 ng/mL IFN-γ in response to the PWM mitogen or greater than 5 ng/mL in the PBS control were considered indeterminate. These criteria resulted in two cattle with indeterminate results because of low IFN-γ production, even after stimulation with the PWM mitogen, and another that showed IFN-γ production without antigenic stimulation. Therefore, these three cattle were removed from the subsequent analyses.
A receiver operating characteristics (ROC) curve was generated for the IFN-γ assay using the ESAT-6 and CFP-10 antigen cocktail to determine the cutoff value for an IFN-γ-positive result (Fig. 3). The most appropriate cutoff was defined as the point on the ROC curve with the greatest distance from the diagonal line (sensitivity = 1 - specificity), which resulted in an appropriate cutoff IFN-γ concentration of 0.125 ng/mL. However, 0.2 ng/mL of IFN-γ was used instead to improve the specificity of the IFN-γ assay without losing much sensitivity. When this cutoff was applied, 116 (85.9%) of the 135 SIDT-positive animals gave IFN-γ assay-positive results, indicating that the assay sensitivity was 85.9%. Additionally, none of the 100 SIDT-negative cattle from the BTB-free herds gave IFN-γ-positive results, indicating that its specificity was 100%.
Only SIDT-positive animals were selectively culled during annual screening; therefore, we investigated the extent of undetected M. bovis infection in the remaining cattle using the IFN-γ assay described above. To accomplish this, we tested a total of 374 animals from two groups of herds that had undergone BTB outbreaks, one consisting of 260 SIDT-negative cattle from 11 dairy herds that suffered BTB outbreaks as determined by the most recent annual test (recent outbreak), and another composed of 114 SIDT-negative cattle from four herds with a history of BTB outbreaks, but in which all of the animals tested negative in the most recent annual test (remote outbreak). The mean IFN-γ concentration of the animals from the herds with remote BTB outbreaks was significantly higher than that of animals from the herds with recent BTB outbreak (data not shown). When the cutoff criteria set by this study were applied for the IFN-γ assay, 79 (30.4%) of the 260 SIDT-negative cattle from herds with recent BTB outbreaks and 42 (36.8%) of 114 SIDT-negative animals from herds with BTB outbreaks that occurred at least 2 months previously tested positive for IFN-γ, respectively (Table 1). These results indicate that a substantial portion of animals had an infection that was not detected by the annual SIDT screen. In addition, although there was no significant difference between groups, there appeared to be a trend towards a greater number of M. bovis infections over time. However, there was marked variation in M. bovis infection rates among dairy cattle herds, regardless of the number of SIDT-positive animals (Table 2). In herd B with a recent BTB outbreak, only one (3.7%) of 27 cattle was SIDT-positive, while 20 (74.1%) were IFN-γ-positive; thus, 19 animals with M. bovis infection were not detected by SIDT. Conversely, six (16.2%) of 37cattle in herd H with a recent BTB outbreak were SIDT-positive, while seven (18.9%) of 37 cattle were IFN-γ-positive; thus, only one additional animal was identified by the developed assay. Based on the results above, total depopulation of animals in herds that have had a BTB outbreak is more suitable as a control practice.
To confirm M. bovis infection among SIDT-negative, but IFN-γ-positive cattle, we slaughtered 14 animals and examined them for the presence of visible lesions. Additionally, we removed the hilar lymph nodes for culture tests and molecular detection of M. bovis (Fig. 4). No visible lesions were found in the internal organs (including the lung, spleen, liver, and kidney), but six cattle had granuloma lesions in their hilar lymph nodes. Additionally, M. bovis was isolated from the hilar lymph nodes of five cattle, four of which had a caseous lesion. Eleven cattle, including six with caseous lesions, were M. bovis-specific IS1081 PCR positive, confirming that the IFN-γ assay used in this study could detect M. bovis in a portion of dairy cattle that were SIDT negative (Table 3).
This study demonstrated that an IFN-γ assay using the ESAT-6 and CFP-10 antigen cocktail is useful for detecting M. bovis infection among dairy cattle with a sensitivity of 86% and a specificity of 100% when compared to SIDT. Although this study was limited in that it used the SIDT results as the criteria for M. bovis infection instead of culture results, the IFN-γ assay results obtained in this study were comparable to those obtained in other studies. For example, a study of 1,479 cattle from herds with BTB outbreaks in Spain revealed that the IFN-γ assay was positive in 149 (85.6%) of 174 SIDT-positive cattle and negative in 1,194 (91.5%) of 1,305 SIDT-negative cattle [5]. In another study of 220 cattle at high risk of BTB in Brazil, all of the 106 SIDT-positive cattle were also positive for IFN-γ, representing a sensitivity of 100%, and there were 20 additional cattle that were SIDT-negative, but IFN-γ assay-positive. Of these 20 animals, 14 were either culture positive or became SIDT-positive upon follow up tests [7]. Therefore, the results obtained by the IFN-γ assay in this study were comparable to those employed in other studies.
In this study, we used the M. tuberculosis complex-specific antigens, ESAT-6 and CFP-10, to reduce false-positive results. During early development of the IFN-γ assay, the PPD-B and PPD-A antigens were used to increase specificity, but they resembled those of the comparative cervical tuberculin test [16,20,21]. However, owing to the availability of M. tuberculosis complex-specific antigens, there have been efforts to develop an IFN-γ assay with higher sensitivity and specificity using the ESAT-6, CFP-10, and other RD1 antigens [11,13]. For example, the ESAT-6 antigen alone gave a comparable result to PPD-B in an in vitro IFN-γ assay of 19 animals infected experimentally with M. bovis [14]. In an extensive analysis of various M. tuberculosis complex-specific antigens, ESAT-6/CFP-10 had the greatest sensitivity (85%), and a specificity of 97% [1]. Use of the ESAT-6 antigen in the IFN-γ assay also gave a higher specificity than that achieved using the PPD-D/PPD-A-based IFN-γ assay (100% vs. 94%, respectively) [2]. Therefore, the IFN-γ assay established in this study produces results comparable to those employed in other studies.
Possibly the most important finding in this study is that more than 30% of SIDT-negative cattle were positive based on IFN-γ assay of herds that had suffered recent BTB outbreaks. These findings suggest that selective culling of SIDT-positive animals under these circumstances is inadequate because it leaves a substantial portion of animals with M. bovis infection, which may act as sources of infection to other animals in the herds. The greater proportion of cattle testing positive presumably reflects the higher sensitivity of the IFN-γ assay than the SIDT. This greater sensitivity of the IFN-γ assay for detection of M. bovis infection is concordant with the findings of a number of previous studies. For example, in a study of 1,362 cattle from M. bovis-infected herds, the IFN-γ assay had a sensitivity of 82% and specificity of 99%, both of which were higher than those of SIDT, for which the sensitivity and specificity were 68% and 97%, respectively [20]. This greater sensitivity of the IFN-γ assay may reflect the fact that the IFN-γ response occurs at an early stage of M. bovis infection, while the changes that define a positive SIDT result only become apparent later. This assumption is supported by an experimental infection of cattle with M. bovis in which an increase in IFN-γ was detected as early as 2 weeks after infection in some animals, and all cattle were positive 4 weeks after infection [15]. However, under natural conditions, the infection dose may vary considerably, along with the time required for a positive IFN-γ assay or SIDT result. In a field study, IFN-γ detected changes 90 ~ 50 days earlier than the SIDT [7]. This may help explain our finding that IFN-γ positivity was slightly higher among the SIDT-negative cattle from herds with earlier BTB outbreaks (36.8%) than herds in which the outbreaks were more recent (30.4%). Thus, the IFN-γ assay may be more effective at detecting M. bovis infections than SIDT in herds with BTB outbreaks.
In an attempt to demonstrate that there was a definite M. bovis infection among SIDT-negative, but IFN-γ positive cattle, we found that 11 (78.6%) of 14 cattle with these test results showed evidence of M. bovis infection either by culture tests (5 animals; 35.7%) or the presence of M. bovis DNA as determined using a PCR-based assay. Although the numbers were small, these findings still clearly demonstrate that the IFN-γ assay can detect genuine M. bovis infections in the majority of SIDT-negative animals. This finding is also supported by those of previous studies. In one such study, 23 (43.4%) of 53 cattle that were IFN-γ-positive but SIDT-negative were found to be culture positive for M. bovis [20], while in other studies, 34% ~ 38% of IFN-γ-positive but SIDT-negative animals were positive for M. bovis culture [12,17]. Therefore, the IFN-γ assay using the ESAT-6 and CFT-10 antigen cocktail employed in this study is considered to be specific for detection of M. bovis infection, even in SIDT-negative cattle.
Taken together, our findings suggest that the IFN-γ assay described in this study is an effective test for M. bovis infection in cattle. Furthermore, if the results of this assay had been applied in addition to the standard SIDT in annual testing, many more cattle should have been culled. Accordingly, these findings indicate the need for an additional sensitive test(s) for M. bovis infection to enable more effective control of BTB, and that the IFN-γ assay could serve as such a test, particularly in countries where only a selective culling strategy based on SIDT results is in use. The only other practical method of controlling this disease may be the total culling of infected herds, although this would have significant economic consequences.
Figures and Tables
 | Fig. 1Standard curve for IFN-γ enzyme-linked immunosorbent assay showing measurement of recombinant bovine IFN-γ. Linearity was determined at IFN-γ concentrations ranging from 0.078 to 10 ng/mL. |
 | Fig. 2IFN-γ secretion of mycobacterial antigens in SIDT-positive and -negative cattle. ***p < 0.0001. |
 | Fig. 3ROC curve calculated for IFN-γ assay using the ESAT6 and CFP10 antigen cocktail. IFN-γ assay AUC = 0.958, 95% confidence interval (CI) = 0.930 to 0.985. |
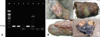 | Fig. 4PCR analysis and visible lesions on hilar lymph nodes of SIDT-negative cattle. (A) Electrophoresis on 1.5% agarose gel showing the 113 bp PCR products after amplification with the IS1081 F/R primers. Lane M, 100 bp DNA size marker; Lane 1, M. bovis DNA; Lanes 2 ~ 7, samples of hilar lymph nodes. (B) Visible lesions of hilar lymph nodes from cattle showing positive response to IFN-γ assay, but negative response to SIDT. |
Table 1
Results of interferon-gamma (IFN-γ) assay of cattle in herds that had bovine tuberculosis (BTB) outbreaks recently and remotely

Acknowledgments
This work was supported in part by a grant from the Korean Health 21 R&D Project, the Ministry of Health and Welfare, Korea (A010381), and a grant from the Brain Korea 21 Project for Medical Sciences at Yonsei University College of Medicine.
References
1. Aagaard C, Govaerts M, Meikle V, Vallecillo A, Gutierrez-Pabello JA, Suarez-Güemes F, McNair J, Cataldi A, Espitia C, Andersen P, Pollock JM. Optimizing antigen cocktails for detection of Mycobacterium bovis in herds with different prevalences of bovine tuberculosis: ESAT6-CFP10 mixture shows optimal sensitivity and specificity. J Clin Microbiol. 2006; 44:4326–4335.

2. Buddle BM, Ryan TJ, Pollock JM, Andersen P, de Lisle GW. Use of ESAT-6 in the interferon-γ test for diagnosis of bovine tuberculosis following skin testing. Vet Microbiol. 2001; 80:37–46.

3. de la Rua-Domenech R, Goodchild AT, Vordermeier HM, Hewinson RG, Christiansen KH, Clifton-Hadley RS. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, γ-interferon assay and other ancillary diagnostic techniques. Res Vet Sci. 2006; 81:190–210.

4. Dillon DC, Alderson MR, Day CH, Bement T, Campos-Neto A, Skeiky YA, Vedvick T, Badaro R, Reed SG, Houghton R. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J Clin Microbiol. 2000; 38:3285–3290.

5. González Llamazares OR, Gutiérrez Martín CB, Alvarez Nistal D, de la Puente Redondo VA, Dominguez Rodríguez L, Rodríquez Ferri E. Field evaluation of the single intradermal cervical tuberculin test and the interferon-γ assay for detection and eradication of bovine tuberculosis in Spain. Vet Microbiol. 1999; 70:55–66.

6. Hope JC, Thom ML, Villarreal-Ramos B, Vordermeier HM, Hewinson RG, Howard CJ. Exposure to Mycobacterium avium induces low-level protection from Mycobacterium bovis infection but compromises diagnosis of disease in cattle. Clin Exp Immunol. 2005; 141:432–439.

7. Lilenbaum W, Schettini JC, Souza GN, Ribeiro ER, Moreira EC, Fonseca LS. Comparison between a γ-IFN assay and intradermal tuberculin test for the diagnosis of bovine tuberculosis in field trials in Brazil. Zentralbl Veterinarmed B. 1999; 46:353–358.

8. Mathews F, Macdonald DW, Taylor GM, Gelling M, Norman RA, Honess PE, Foster R, Gower CM, Varley S, Harris A, Palmer S, Hewinson G, Webster JP. Bovine tuberculosis (Mycobacterium bovis) in British farmland wildlife: the importance to agriculture. Proc Biol Sci. 2006; 273:357–365.

9. Monaghan ML, Doherty ML, Collins JD, Kazda JF, Quinn PJ. The tuberculin test. Vet Microbiol. 1994; 40:111–124.

10. Morris RS, Pfeiffer DU, Jackson R. The epidemiology of Mycobacterium bovis infections. Vet Microbiol. 1994; 40:153–177.
11. Mustafa AS, Cockle PJ, Shaban F, Hewinson RG, Vordermeier HM. Immunogenicity of Mycobacterium tuberculosis RD1 region gene products in infected cattle. Clin Exp Immunol. 2002; 130:37–42.

12. Neill SD, Cassidy J, Hanna J, Mackie DP, Pollock JM, Clements A, Walton E, Bryson DG. Detection of Mycobacterium bovis infection in skin test-negative cattle with an assay for bovine interferon-gamma. Vet Rec. 1994; 135:134–135.

13. Pollock JM, Girvin RM, Lightbody KA, Clements RA, Neill SD, Buddle BM, Andersen P. Assessment of defined antigens for the diagnosis of bovine tuberculosis in skin test-reactor cattle. Vet Rec. 2000; 146:659–665.

14. Pollock JM, Andersen P. Predominant recognition of the ESAT-6 protein in the first phase of interferon with Mycobacterium bovis in cattle. Infect Immun. 1997; 65:2587–2592.

15. Rhodes SG, Palmer N, Graham SP, Bianco AE, Hewinson RG, Vordermeier HM. Distinct response kinetics of gamma interferon and interleukin-4 in bovine tuberculosis. Infect Immun. 2000; 68:5393–5400.

16. Rothel JS, Jones SL, Corner LA, Cox JC, Wood PR. The gamma-interferon assay for diagnosis of bovine tuberculosis in cattle: conditions affecting the production of gamma-interferon in whole blood culture. Aust Vet J. 1992; 69:1–4.

17. Scacchia M, Lelli R, Petrini A, Prencipe V, Calistri P, Giovannini A. Use of innovative methods in the eradication of bovine tuberculosis. J Vet Med B Infect Dis Vet Public Health. 2000; 47:321–327.

18. Taylor GM, Worth DR, Palmer S, Jahans K, Hewinson RG. Rapid detection of Mycobacterium bovis DNA in cattle lymph nodes with visible lesions using PCR. BMC Vet Res. 2007; 3:12.

19. Wang BL, Xu Y, Wu CQ, Xu YM, Wang HH. Cloning, expression, and refolding of a secretory protein ESAT-6 of Mycobacterium tuberculosis. Protein Expr Purif. 2005; 39:184–188.

20. Wood PR, Corner LA, Rothel JS, Baldock C, Jones SL, Cousins DB, McCormick BS, Francis BR, Creeper J, Tweddle NE. Field comparison of the interferon-gamma assay and the intradermal tuberculin test for the diagnosis of bovine tuberculosis. Aust Vet J. 1991; 68:286–290.

21. Wood PR, Corner LA, Rothel JS, Ripper JL, Fifis T, McCormick BS, Francis B, Melville L, Small K, de Witte K, Tolson J, Ryan TJ, de Lisle GW, Cox JC, Jones SL. A field evaluation of serological and cellular diagnostic tests for bovine tuberculosis. Vet Microbiol. 1992; 31:71–79.

22. Wood PR, Jones SL. BOVIGAM™: an in vitro cellular diagnostic test for bovine tuberculosis. Tuberculosis (Edinb). 2001; 81:147–155.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download