Introduction
Infectious bronchitis virus (IBV), a member of the coronavirus family, is prevalent in all countries with an intensive poultry industry [8]. IBV is highly infectious and spreads rapidly in unprotected birds [32]. IBV replicates in the epithelium of respiratory tract tissues, as well as in many non-respiratory tissues, such as those from kidneys and gonads [5,29]. There are a large number of IBV variants worldwide, and they cause great economic losses to the poultry industry.
Vaccination is the most effective method of preventing virus infection and controlling the spread of IBV. Both inactivated and live attenuated virus vaccines have been used to protect chickens against IBV [4,9]. Inactivated virus vaccines are produced from IBV-infected allantoic fluids, but do not illicit high levels of S1-specific antibody responses [33]. Live attenuated viruses offer long lasting and protective immunity, but pose the risk of returning to virulent form and co-infection with Escherichia coli or other bacteria [10,34]. Accordingly, it is necessary to develop a new IBV vaccine.
Spike (S) protein, one of the four structural proteins of IBV, is a heavily glycosylated spike glycoprotein expressed on virion surfaces [13]. This protein be cleaved into two subunits, N-terminal S1 and C-terminal S2. The S1 subunit, which is the bulbous head of the S protein, is responsible for attachment of the virus to cells [7]. Analysis of S-specific monoclonal antibodies has shown that many of the amino acids of virus neutralization (VN) epitopes are located within the first and third quarters of the linear S1 polypeptide [11,12,19]. Immune responses induced by the S1 subunit have been studied using S1 protein prepared from purified virus and derived from baculovirus-based expression systems [16,23,33].
Virus-like particles (VLPs) are multi-protein structures that mimic the organization and conformation of authentic native viruses without viral genomes. VLPs are generated by assembling structural viral proteins and lipids into particles [18,30]. VLPs have been widely investigated for use in the development of safe and effective vaccines because the viral antigens on the surfaces of VLPs can induce humoral and cellular responses [24,26,27]. Two VLP-based vaccines have already been licensed for use in humans against hepatitis B virus and HPV, and more VLP-based vaccines are being evaluated in preclinical and clinical trials. In addition, chimeric VLPs have been generated by substituting part or all of the extracellular domain of a surface antigen of a VLP derived from one virus with one from another virus, and these VLPs have been shown to induce immune responses against the surface antigen from the other virus [35].
VLPs based on IBV structural proteins have been reported and employed for investigations of protein-protein interactions and assembly of virons [2,11,17,22,31]. Influenza virus is a major threat to human health that causes significant morbidity and mortality worldwide and is therefore always at the forefront of vaccine research. Influenza VLPs have been generated by co-infecting insect cells with recombinant baculoviruses expressing structural influenza proteins of matrix 1 (M1)/hemagglutinin (HA), HA/neuraminidase (NA)/M1, or HA/NA/M1/matrix 2 (M2). [14,15,21,28,33]. Influenza VLPs have been found to induce protective immunity in preclinical and clinical studies [20]. In light of the above findings, this study was conducted to investigate whether influenza VLPs could serve as a platform for the expression of IBV S1 protein, and whether VLPs containing S1 protein could serve as a candidate IBV vaccine.
In this study, we generated a fusion protein in which the IBV S1 protein was fused to the cytoplasmic tail (CT) and the transmembrane (TM) domain of avian influenza H5N1 virus NA protein. The results showed that the fusion protein and avian influenza virus M1 protein were efficiently assembled to form chimeric VLPs. These chimeric VLPs were then prepared quantitatively and used as immunogens in BALB/c mice and SPF chickens. When compared with the IBV inactivated vaccine, the chimeric VLPs induced higher immune responses. Taken together, the chimeric VLPs showed the potential for use as a candidate vaccine against IBV.
Materials and Methods
Cell line and virus
Spodoptera frugiperda Sf-9 cells were maintained in Grace's insect cell culture medium (Gibco, USA) supplemented with 10% heated-inactivated fetal bovine serum (FBS), 100 µg/mL streptomycin and 100 IU/mL penicillin in a 27℃ humidified incubator. IBV strain H120 (accession number M21970) was propagated in 9-day-old specific pathogen free (SPF) embryonated chicken eggs.
Construction of recombinant baculoviruses
Briefly, the genes encoding NA and M1 proteins of influenza virus A/GOOSE/GD/96 (H5N1; Access No. NC_007363) and S1 protein of IBV H120 were first obtained by RT-PCR (PrimeScripTM 1st Strand cDNA Synthesis Kit, Takara Bio, China) and then cloned into pMD-18T vector to obtain recombinant plasmids pMD-18T-NA, pMD-18T-M1 and pMD-18T-S1. NA/S1 fusion gene was then generated by overlap PCR. The full-length of the fusion gene was 1674 bp, and it contained the CT and TM domains of NA (1~120 bp) and the S1 sequence (121~1,674 bp) [3]. The specific primers used to amplify the CT and TM domain were CCGGAATTCATGAATCCAAATCAGAA (forward primer 1) and GACCCATATTGAGATTAGTTTTGTATG (reverse primer 1). The primers used to generate S1 were CAATATGGGTCATGTCGTACTACCATC (forward primer 2) and ACGCGTCGACACGTCTAAAACGACGTGTT (reverse primer 2).
The above two PCR products were mixed equally to generate the fusion gene NA/S1 by PCR using forward primer 1 and reverse primer 2. The nucleotide sequences of the NA/S1 fusion and M1 genes were confirmed by DNA sequencing and then cloned into pFast-Bac-Dual vector (Invitrogen, USA) [26]. Recombinant bacmids r-Bacmid-NA/S1 and r-Bacmid-M1 were generated by transforming the recombinant pFast-Bac-Dual vector into Escherichia coli DH10-Bac competent cells. The purified recombinant bacmid DNA was then transfected into sf9 cells with cellfectin reagent (Invitrogen). After 3 days, the recombinant baculoviruses (rBV-NA/S1 and rBV-M1) were obtained from the supernatant and subjected to three rounds of plaque purification.
Western blot
Sf9 cells at a density of 1 × 106 per flask were infected with rBV-NA/S1 and rBV-M1 at a multiplicity of infection (MOI) of 5. After 72 h, a marked cytopathic effect was observed in the infected cells. These infected cells were harvested and then analyzed by western blot. Briefly, samples were resolved on 8% gradient polyacrylamide gels, after which fusion protein was detected with chicken polyclonal sera against IBV H120 virus and the M1 protein was detected with chicken polyclonal sera against influenza virus H5N1. Horseradish peroxidase (HRP)-conjugated donkey anti-chicken secondary antibody (Proteintech Group, USA) was used at a dilution of 1 : 5,000. Sf9 cells were infected with the same amount of wild-type baculovirus and treated in parallel with the recombinant baculovirus infected cells to be used as a negative control.
Indirect immunofluorescence
Sf9 cells were grown on 24 × 24 mm glass coverslips in 6-well culture plates and infected with both rBV-NA/S1 and rBV-M1 at a MOI of 5 for 48 h and then fixed. The rBV-M1 infected cells were treated with 0.3% TrintonX-100, while control cells were infected with wild-type baculoviruses and processed in parallel with other samples. The expression of fusion protein NA/S1 was detected by chicken polyclonal sera against IBV H120 virus and FITC-conjugated donkey anti-chicken secondary antibody (FITC, green; Beijing Biosynthesis Biotechnology, China). M1 protein was detected by mouse polyclonal sera against influenza virus H5N1 and CY3-conjugated rabbit anti-mouse secondary antibody (CY3, red; Beijing Biosynthesis Biotechnology). The nucleus was stained with DAPI (Beijing Biosynthesis Biotechnology). Coverslips were mounted on glass slides and analyzed on a Leica TCS-SP5 confocal laser scanning microscope.
Preparation of chimeric VLPs
To produce chimeric VLPs containing the fusion proteins NA/S1 and M1, sf9 cells were co-infected with recombinant baculoviruses expressing NA/S1 and M1 at a MOI of 5. Culture supernatants were harvested at 72 h post-infection. After removing cells by centrifugation at 2,000 × g for 30 min, the VLPs in the supernatants were pelleted by ultracentrifugation at 80,000 × g for 2 h and then resuspended in PBS. The particles were further purified through a 20-30-40-50% discontinuous sucrose gradient at 80,000 × g for 2 h at 4℃, after which the purified VLPs were collected and the presence of fusion protein NA/S1 and M1 protein was analyzed by western blot as described above.
Electron microscopy
The purified VLPs were analyzed by electron microcopy as previously described [21]. Briefly, 200-mesh carbon-coated copper grids were floated on drops of chimeric VLPs for 10 min, then washed with water three times. Next, 2% phosphotungstic acid (PH 7.0) was applied for 1 min for negative staining. After the girds were air-dried, the samples were examined by transmission electron microscopy (JEM-100CX-II; JEOL, Japan) at a magnification of 20,000×.
Animal immunization
Three groups of 6-week-old female BALB/c mice (n = 6) were intramuscularly (i.m.) immunized with chimeric VLPs, inactivated IBV H120 viruses or PBS three times (week 0, 2 and 4) at two-week intervals. Each dose of chimeric VLPs or inactivated IBV H120 viruses contained 2 µg S1 proteins plus complete Freund's adjuvants (CFA) for the primary immunization, or incomplete Freund's adjuvant (IFA) for subsequent boosts. Serum samples were collected before immunization and at 14 days after each immunization, then stored at -80℃ until analysis.
Three groups of 10-day-old SPF chickens (n = 20) were i.m. immunized with chimeric VLPs, inactivated IBV H120 viruses or PBS twice (week 0 and 2) at a two-week interval. Each dose of chimeric VLPs or inactivated IBV H120 viruses contained 2 µg of S1 protein plus MONTANIDE ISA 206 (SEPPIC, France) as an adjuvant. Serum samples were collected before immunization and at 14 days after each immunization and stored at -80℃ until analysis.
ELISA
Serum S1-specific antibodies were analyzed by Enzyme-Linked Immunosorbent Assay (ELISA). Briefly, S1 proteins were purified from 293T cells expressing S1 proteins. Each well of a 96-well plate was then coated with 100 µL of S1-proteins at 3 µg/mL. After washing and blocking, each well was incubated with serum samples from immunized mice or from immunized SPF chickens at a 400-fold dilution for 2 h. Following washing, horseradish peroxidase (HRP)-labeled rabbit anti-mouse secondary antibodies (Proteintech Group) diluted 5000-fold were added and incubated for 1 h for murine sera. For chicken sera, HRP-labeled donkey anti-chicken secondary antibodies (Proteintech Group) diluted 5000-fold were added and incubated for 1 h. Signals were visualized with ELISA substrate reagents and measured at 450 nm using a spectrophotometer (ELx800UV; BioTek, USA).
Cytokine assay
Murine spleens were collected from sacrificed mice 14 days after the third immunization. Murine splenocytes were obtained using mouse 1× lymphocyte separation medium (Dakewe Biotech, China). The numbers of specific cells that secreted murine IFN-γ (mIFN-γ) or murine IL-4 (mIL-4) were determined by ELISPOT assay (R&D Systems, USA). Splenocytes were stimulated by purified IBV H120 viral particles at a concentration of 10 µg/mL. The number of spots was counted by an ImmunoSpot ELISPOT reader (Bioreader4000; BIO-SYS, Germany).
Virus neutralization assay
Serum samples were heat-inactivated at 56℃ for 30 min, then two-fold serially diluted. The diluted serum samples were mixed with IBV H120 viruses (100xTCID50; TCID, tissue culture infective dose) and incubated at 37℃ under 5% CO2 for 1h. The mixture of serum and H120 virus was inoculated into 9-day-old SPF embryonated chicken eggs. The neutralization titers were expressed as the reciprocal of the highest serum dilution that neutralized 100xTCID50 IBV H120 in 50% of the SPF embryonated chicken eggs.
Results
Expression of NA/S1 fusion protein and M1 protein in sf9 cells
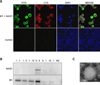
The fusion protein NA/S1 was composed of S1 protein (521 amino acids without signal peptide) of H120 S protein and the CT and TM domain (40 amino acids) of the NA protein from H5N1 (Fig. 1A). The estimated molecular weight (MW) was about 100 KD for NA/S1 fusion protein and 25 KD for M1 protein. Western blot analysis showed that NA/S1 was detected as a 100 KD band (Fig. 1B), while M1 protein appeared as a 25 KD band (Fig. 1C). It should be noted that one faint 100 KD band was detected together with M1 protein when probed with chicken polyclonal antibodies against whole influenza H5N1 virus, suggesting that the chicken polyclonal antibodies could react with the CT and TM domains of NA protein. Nevertheless, these results demonstrated that NA/S1 and M1 were properly expressed in Sf9 cells.
Production and analysis of chimeric VLPs
Indirect immunofluorescence assay confirmed the co-expression of NA/S1 (FITC) and M1 (CY3) proteins in the co-infected cells (Fig. 2A). Chimeric VLPs were generated by co-infection of sf9 cells with both rBV-NA/S1 and rBV-M1. To optimize the production of chimeric VLPs, varied combinations of rBV-NA/S1 and rBV-M1 with different MOIs (1/1; 1/5; 1/10; 5/5; 5/1; 10/1) were used to co-infect cells, after which the yields of VLPs were determined. The results showed that the co-infection of both rBV-NA/S1 and rBV-M1 at an MOI of 5 generated the optimal yield (Fig. 2B). The chimeric VLPs were purified by discontinuous sucrose density gradients, after which electron microscopy was used to examine the formation of chimeric VLPs. The observed VLPs had diameters of about 100 nm, showing morphological characteristics similar to the standard influenza VLPs (Fig. 2C).
Humoral immune responses induced by chimeric VLPs in mice
Mouse serum samples were immunized with chimeric VLPs and inactivated IBV H120 viruses (diluted 400 times) and then analyzed for their S1-specific IgG antibodies by ELISA. Purified S1 protein was selected for the ELISA experiments because S1 protein was a common surface antigen between chimeric VLPs and H120 viruses; therefore, measurement of S1-specific IgG antibodies should make the results from both groups more comparable. In addition, since there were no available standard antigens, the quantification of S1-specific antibodies was difficult; accordingly, the OD was used for comparison. In both groups, each boost increased S1-specific antibody responses. Additionally, chimeric VLPs induced higher S1-specific antibody responses than inactivated IBV H120 viruses (p < 0.01) after the first boost (Fig. 3).
Cellular immune responses induced by chimeric VLPs in mice
Cellular immune responses were evaluated through detection of the number of splenocytes secreting IFN-γ or IL-4 using the ELISPOT assay. Single-cell suspensions of splenocytes prepared from the spleens of thrice-vaccinated mice were stimulated by inactivated H120 virus. The results showed that chimeric VLPs elicited higher levels of IL-4 production than inactivated H120 viruses (p < 0.01; Fig. 4A), while both induced comparable IFN-γ production (Fig. 4B), suggesting that the chimeric VLPs could elicit both Th1 and Th2 type cellular immune responses in mice.
IBV-specific neutralization antibodies elicited by VLPs in mice
The IBV-specific neutralization antibody titers were measured in serum samples collected 14 days after the second boost. Both chimeric VLP and inactivated H120 virus groups had higher neutralization titers than the PBS group (p < 0.01; Fig. 5). However, the chimeric VLPs and inactivated H120 groups did not differ significantly (p > 0.05), although the VLP group had a slightly higher neutralizing antibody titer.
Humoral immune responses induced by chimeric VLPs in SPF chickens
Serum S1-specific IgG antibody titers were determined by ELISA, which showed that chimeric VLPs were as efficient at stimulating total anti-S1 antibodies as inactivated viruses (Fig. 6). The chimeric VLP group and the inactivated H120 viruses group both exhibited a higher level of antibodies than the PBS control group (p < 0.01). Additionally, the level of antibodies of the chimeric VLP group was higher than that of the H120 group (p < 0.05).
IBV-specific neutralization antibodies elicited by chimeric VLPs in SPF chicken
Serum IBV-specific neutralization antibody titers in chickens were measured in serum samples from vaccinated SPF chickens 14 days after boost immunization. When compared with the PBS group, chimeric VLPs and inactivated H120 viruses groups had higher neutralizing antibody titers (p < 0.01). The chimeric VLPs were higher than the inactivated H120 groups (p < 0.01; Fig. 7).
Discussion
In this study, we investigated whether chimeric VLPs expressing IBV S1 protein on their surfaces could be generated in sf9 cells. The results showed that chimeric VLPs could be assembled by co-expressing fusion protein NA/S1 and influenza virus M1 protein. We also provided evidence demonstrating that chimeric VLPs were immunogenic and induced S1-specific antibody responses at a higher level than the inactivated H120 viruses. Furthermore, chimeric VLPs could induce significantly higher cellular immune-responses than the inactivated H120 viruses.
IBV has an enormous economic impact on commercial poultry by causing highly contagious disease in chickens of all ages. Vaccination has been used to control IB for over half a century. Although inactivated and attenuated virus vaccines have contributed immensely to control of IB, they have some drawbacks. For example, attenuated virus vaccines pose a potential hazard of propagation of the virus via embryonated chicken eggs and therefore the risk of spreading the virus and generation of a stronger form. Despite this risk, inactivated virus vaccines are commonly used in combination with attenuated virus vaccines because they cannot induce a high level of S1-specific antibodies [10,16,21,25].
Chimeric VLP is a promising development in the field of VLP research. For example, Newcastle disease VLPs contain respiratory syncytial virus proteins [26], while membrane-anchored flagellin were incorporated into influenza VLPs [6]. In the present study, we demonstrated that fusion protein could successfully assemble and bud with influenza M1 to form chimeric VLPs, and that chimeric VLPs were similar to previously reported influenza VLPs. Accordingly, the results presented herein provide an additional example supporting the feasibility of generating chimeric VLPs [1].
This study demonstrated that the chimeric VLPs induced robust humoral responses with an obviously higher level than inactivated H120 viruses in both mice and chickens (p > 0.05). In addition, a higher level of IL-4 was detected in the VLPs group than in the inactivated H120 group (p < 0.05). Although the mechanism by which the chimeric VLPS elicited significantly higher IL-4 production than the inactivated H120 viruses is not clear. Furthermore, chimeric VLPs induced significantly higher neutralizing antibody titers than inactivated IBV viruses.
In summary, this study demonstrated the generation of a chimeric VLP by expression of the IBV S1 protein on the surface of influenza VLPs. The results showed that the chimeric VLPs could elicit humoral and cellular immune responses and neutralization antibody response at a level significantly higher than that of inactivated IBV viruses. Accordingly, chimeric VLPs are a potential candidate for the development of vaccines against IBV.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


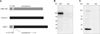



 XML Download
XML Download