Abstract
Currently, killed-virus and modified-live porcine reproductive and respiratory syndrome virus (PRRSV) vaccines are used to control porcine reproductive and respiratory syndrome. However, both types of vaccines have inherent drawbacks; accordingly, the development of novel PRRSV vaccines is urgently needed. Previous studies have suggested that yeast possesses adjuvant activities, and it has been used as an expression vehicle to elicit immune responses to foreign antigens. In this report, recombinant Kluyveromyces lactis expressing GP5 of HP-PRRSV (Yeast-GP5) was generated and immune responses to this construct were analyzed in mice. Intestinal mucosal PRRSV-specific sIgA antibody and higher levels of IFN-γ in spleen CD4+ and CD8+ T cells were induced by oral administration of Yeast-GP5. Additionally, Yeast-GP5 administered subcutaneously evoked vigorous cell-mediated immunity, and PRRSV-specific lymphocyte proliferation and IFN-γ secretion were detected in the splenocytes of mice. These results suggest that Yeast-GP5 has the potential for use as a vaccine for PRRSV in the future.
Porcine reproductive and respiratory syndrome (PRRS) is an important disease in pigs that causes tremendous economic losses to the swine industry worldwide. The causative agent, PRRS virus (PRRSV), is an enveloped, single-stranded positive RNA virus belonging to the genus Arterivirus, family Arteriviridae [38]. In April 2006, atypical PRRS characterized by high fever, high morbidity, and mortality emerged in China, affecting more than 20 million pigs of all ages [22]. The causative agent was a highly pathogenic PRRSV (HP-PRRSV) genotype with a discontinuous deletion of 30 amino acids in nonstructural protein 2 (NSP2) [37].
At present, two types of commercial vaccines against PRRSV are available, modified live-attenuated vaccines (MLVs) and inactivated vaccines [19]. MLVs confer some protection against clinical diseases induced by homologous infection; however, they have been found to be associated with numerous problems including shedding of vaccine virus, persistent infection, and reversion to virulence [10]. Killed-virus vaccines are considered to be ineffective for stimulation of cell-mediated immunity and fail to establish protective immunity [39]. It should be noted that, when the highly pathogenic PRRSV emerged in China, the currently used commercial vaccines provided limited protection against HP-PRRSV epidemics. Since then, great efforts have been made to develop vaccines against HP-PRRSV. Genetically engineered PRRSV vaccines, including recombinant vectors expressing PRRSV viral proteins, DNA vaccines and plant-made subunit vaccines, have been developed and tested against PRRSV. Those recombinant vectors expressing PRRSV viral proteins include recombinant adenovirus or fowlpox virus co-expressing GP3 and GP5 [28,35], recombinant pseudorabies virus expressing GP5 [26], mycobacterium bovis BCG expressing GP5 and M [5], recombinant DNA vaccines expressing GP5 [21], corn plants expressing PRRSV M protein [17], and tobacco plant expressing GP5 [12]. All of these vaccines have their own potential and limitations.
PRRSV has eight viral structural proteins and 14 non-structural proteins. PRRSV GP5 protein encoded by ORF5, which is one of the most abundant viral antigens on the viral envelope, contributes to the entry of PRRSV into cells [31]. One neutralizing epitope and two T cell epitopes have been identified within this protein [25,34], and most of the neutralizing antibodies are predominantly directed against GP5 [24]. These characteristics make GP5 a promising candidate for the development of PRRSV vaccines.
The yeast system has been shown to have advantages over conventional systems as a vaccine vehicle [3]. For example, Saccharomyces (S.) cerevisiae is generally regarded as safe (GRAS) for animals and human beings. Furthermore, studies have demonstrated that yeast cell wall components possess multiple adjuvant properties and are able to activate the immune system [2,15]. However, there are some limitations to S. cerevisiae expression systems. Specifically, S. cerevisiae has a tendency to hyperglycosylate recombinant proteins, and N-linked carbohydrate chains are terminated with alpha-1,3-linked mannose residues, which are considered to be allergenic. Kluyveromyces (K.) lactis, one of the most important non-Saccharomyces yeasts, has similar advantages as S. cerevisiae, as well as the potential to overcome the described limitations [9,32]. Additionally, K. lactis has a well-established track record of safe use in various food industry applications and can efficiently express heterologous proteins. Moreover, components of its cell-wall such as β-1,3-glucan and mannan may have adjuvant activities. Thus, K. lactis might be a safe and ideal vaccine vehicle.
The mucosa of respiratory and reproductive tracts is the major route of PRRSV infection [33]. It is believed that generating mucosal immunity using vaccines is the best way to prevent PRRSV infection. It has been reported that recombinant yeast can be administered orally and efficiently taken up by M cells, after which it delivers proteins to antigen presenting cells (APCs) in Peyer's patches to induce mucosal immune responses [8,29]. Moreover, vaccination subcutaneously (sc) with recombinant S. cerevisiae expressing several different antigens has been shown to induce antigen-specific T-cell responses both in vitro and in vivo [3,6,14,27].
In the present study, we constructed recombinant K. lactis expressing HP-PRRSV GP5 and evaluated its ability to induce B cell- and T cell- mediated immune responses in BALB/c mice immunized orally and subcutaneously, respectively.
Porcine alveolar macrophages (PAMs) were obtained by postmortem lung lavage of 8-week-old specific pathogen free (SPF) pigs and maintained in RPMI 1640. PRRSV strain JXwn06, which was isolated from a pig farm with an atypical PRRS outbreak in 2006 and identified as a HP-PRRSV, was propagated and titrated on PAMs.
JXwn06 was propagated in PAMs and purified by sucrose density gradient separation. Briefly, the virus was concentrated from the supernatant by ultracentrifugation at 120,000 × g for 2 h and 4℃. The virus pellet was then resuspended in phosphate buffered saline (PBS), layered on 30% and 60% (wt/vol) sucrose gradients, and centrifuged at 100,000 × g for 2 h and 4℃. The purified virus band was collected and resuspended in PBS, after which it was centrifuged at 120,000 × g for 2 h and 4℃ to pellet the purified virus. Finally, the purified-PRRSV antigen was resuspended in PBS and stored at -80℃.
Recombinant K. lactis constructs without antigen (control yeast, Yeast-vector), with PRRSV GP5 (Yeast-GP5), or with GP5-His (Yeast-GP5-His) were engineered using pKLAC1 vector (New England Biolabs, USA). Briefly, viral RNA was extracted from JXwn06 strain-infected PAMs using TRIzol reagent (Invitrogen, USA) according to the manufacturer's protocols. PRRSV GP5 and GP5-His were then amplified by RT-PCR from viral RNA with the specific primers (sense: GP5-1, and antisense: GP5-2 and GP5-3) listed in Table 1, then subcloned into pKLAC1 at the XhoI and BglII sites. The constructed plasmids pKLAC1-GP5, pKLAC1-GP5-His, and empty vector pKLAC1 were subsequently digested with BstXI to create a linear expression cassette, after which they were transformed into competent K. lactis GG799 by electroporation. The transformants were then cultured in selection medium YCB with 5 mM acetamide and screened by PCR. Recombinant yeast constructs were screened by whole-cell PCR with Integration Primers 1, 2, and 3 (P1, P2 and P3) (Table 1). Single-copy or tandem-vector integration at the LAC4 locus was detected by PCR using P1 and P2 to amplify a 1.9 kb fragment, whereas multi-copy integration was detected using P2 and P3 to amplify a 2.3 kb fragment. The recombinant K. lactis were then grown in YPGal medium (1% yeast extract, 2% bacto-peptone, and 2% lactose) with shaking (~250 rpm) for 3 days at 30℃. The expression products were analyzed by western blot. Yeast-GP5 and Yeast-vector were washed three times with sterile PBS (pH 7.4), heat-killed for 2 h at 60℃, and then stored at -80℃ until use. The Yeast-GP5 and Yeast-vector strains were prepared and maintained in an identical manner.
Recombinant yeasts grown in YPGal were harvested by centrifugation when the culture reached an optical density of 15 at 600 nm. Next, the yeast pellets were lysed by post-alkaline extraction as described previously [20], then separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membrane (Millipore, USA). After blocking with 5% skim milk in PBS with Tween-20 (PBST 0.05% Tween-20), the membranes were incubated for 1 h with rabbit anti-GP5 serum (made in our lab, 1 : 2,000). Specific reaction products were subsequently detected using goat anti-rabbit IgG conjugated with horseradish peroxidase (Santa Cruz Biotechnology, USA) and revealed using a chemiluminescence substrate (EMD Millipore, USA) according to the manufacturer's instructions.
Female BALB/c mice (6-weeks old) were purchased from the Animal Institute of the Chinese Medical Academy (Beijing, China) and randomly divided into four groups (six mice/group). Use of animals was approved by the Institutional Animal Care and Use Committee of China Agricultural University. Mice were immunized with recombinant yeast constructs three times at a one week interval. As shown in Table 2, group 1 and 2 were subcutaneously injected with 200 µL sterile PBS (pH 7.4) containing 2 × 108 Yeast-GP5 or Yeast-vector per mice three times. For oral administration, group 3 and 4 were immunized with 5 × 108 Yeast-GP5 or Yeast-vector in 200 µL sterile PBS (pH 7.4) on day 0, 7 and 14 via gavage with a ball tipped disposable feeding needle.
Serum samples were collected before the first immunization, 1 week after the second immunization (day 14), and 1 week after the last immunization (day 21). Small-intestinal fluids and vaginal lavage fluids were collected at 7 days after the final immunization (day 21). Briefly, a 10-cm-long tissue section was obtained from the small intestine and flushed with 400 µL PBS to recover small-intestinal fluids. The fluids were then vortexed and incubated for 2 h at 4℃, after which they were centrifuged at 8,000 × g for 10 min to eliminate debris. Vaginal lavage fluids were subsequently harvested by rinsing the vaginal cavity with 200 µL PBS and then centrifuged at 8,000 × g for 5 min to remove debris. Serum samples, small-intestinal fluids, and vaginal washes were stored at -20℃ until use. At 7 days after the final immunization, mice were sacrificed and splenocytes were harvested.
PRRSV-specific IgG and sIgA were examined as follows: 96-well ELISA plates were coated with purified-PRRSV antigen at a concentration of 5 µg/mL at 4℃ overnight, then blocked with PBST (PBS containing 0.5% Tween-20) containing 5% skimmed milk for 2 h at 37℃. The sera, small-intestinal washes, and vaginal washes of mice were diluted 1 : 250, 1 : 40, and 1 : 20 in PBST with 2% skimmed milk, then added into the plates. Following incubation for 1 h at 37℃, the coated plates were washed with PBST and incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (1 : 10,000) to detect IgG in sera, or with goat anti-mouse IgA (Southern Biotech, USA) (1 : 4,000) to detect sIgA in mucosal secretions for 1 h at 37℃. After washing with PBST, a color reaction was conducted using tetramethylbenzidine (TMB; Sigma, USA), stopped with 2 M H2SO4, and measured at 450/620 nm using an ELISA reader.
Mice were sacrificed on day 7 after the last immunization and splenocytes were harvested and adjusted to a final concentration of 2 × 106 cells/mL in RPMI 1640. The splenocyte suspensions were then added to 96-well tissue plates (100 µL/well), after which purified-PRRSV antigen (10 µg/mL), ionomycin (500 ng/mL)/PMA (50 ng/mL) or BSA was added. After 60 h of incubation at 37℃ under a 5% CO2 atmosphere, the proliferation responses were detected by MTT (3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide) (5 mg/mL, Sigma) assay [13], and the OD values were read at 490 nm using a plate reader (Magellan; Tecan Austria, Austria). Lymphocyte proliferation was expressed as the stimulation index (SI), which was defined as the ratio of the average OD490 value of stimulated wells to that of un-stimulated wells.
Splenocytes (6 × 106 cells/well) were cultured in 12-well plates and stimulated with purified-PRRSV antigen (10 µg/mL) for 6 h at 37℃. Brefeldin A (BFA; Becton, Dickinson and Company, USA) was added to block the release of cytokine for the last 2 h. Next, the cells were resuspended for immunofluorescent staining. Specifically, the cells were stained with fluorescein isothiocyanate (FITC)-labeled anti-Mouse-CD4 or allophycocyanin (APC)-labeled anti-Mouse-CD8a monoclonal antibodies in the dark for 30 min at 4℃. After washing with PBS, the cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% saponin at room temperature, then stained with phycoerythrin (PE)-labeled anti-mouse-IFNγ, anti-mouse-IL4 antibody, or Rat IgG1 isotype control (eBioscince, USA) overnight at 4℃. Finally, the cells were washed and analyzed with a FACSCalibur using the Cell Quest Pro Software (BD Bioscience, USA).
The pKLAC1 encoding GP5 was constructed and verified by DNA sequencing, after which the vector was digested with BstXI to create a linear DNA fragment that was inserted into the native LAC4 promoter by homologous recombination in K. lactis GG799 (Fig. 1A). The frequency of correctly targeted vector integration at LAC4 was assessed by whole-cell genomic PCR with Integration Primers. As shown in Figs. 1B and C, recombinant Yeast-GP5 and Yeast-vector yielded 1.9 kb and 2.3 kb fragments when amplified with primers P1/P2 and P2/P3, respectively, indicating that these vectors undergo multicopy integration. Expression of GP5 in recombinant K. lactis was confirmed by western blot using PRRSV GP5-specific antibody (Fig. 1E). Yeast-GP5-His, which was detected by anti-His-tag antibody, was used as a positive control. Two GP5-specific bands were observed in Yeast-GP5, one that was about 30 kDa and another that was approximately 32 kDa. The size of the bands may have differed due to the different glycosylations of GP5 in the yeast expression system. These data suggested that GP5 was successfully expressed in K. lactis.
To examine the humoral responses elicited by Yeast-GP5, serum samples of mice were collected at day 14 and 21 and evaluated by iELISA. As shown in Fig. 2A, PRRSV-specific IgG antibody in serum was weak in mice immunized with Yeast-GP5, both subcutaneously and orally, at day 14 and 21.
For the oral immunization groups, mucosal immune responses were analyzed by iELISA for antigen-specific sIgA in small-intestinal fluids and vaginal washes. Our results showed that the level of PRRSV-specific sIgA in small-intestinal washes of the test group was significantly higher (about 3-fold higher) (p = 0.0015) than that of the control group (Fig. 2B). The level of sIgA in the vaginal washes of the Yeast-GP5 immunized group was significantly higher than that of the Yeast-vector immunized group. Generally, sIgA levels in vaginal washes were lower than in small-intestinal washes. These data indicate that oral immunization with Yeast-GP5 induced mucosal immune responses in mice.
To investigate whether immunization with recombinant K. lactis induced cell-mediated immunity (CMI), a lymphocyte-proliferation assay was performed on day 7 after the third immunization. As shown in Fig. 3, strong proliferative responses were observed in mice immunized subcutaneously with the Yeast-GP5, and an approximately 2-fold increase was observed relative to that in mice immunized with Yeast-vector. There was no T-cell proliferation response in the oral immunized groups. These results suggested that Yeast-GP5 could induce PRRSV-specific T-cell proliferation when delivered subcutaneously.
Cytokines play a pivotal role in the modulation of immune responses. To develop cytokine profiles, we first examined the level of IL-4 (TH2 type) production in CD4+ T cells using a fluorescence activated cell sorter (FACS). As shown in Fig. 4, there was no significant difference in the percentage of IL-4+CD4+ T cells between Yeast-GP5 and Yeast-vector, suggesting that immunization with Yeast-GP5 cannot efficiently stimulate TH2-type cytokine secretion.
We also examined the levels of IFN-γ in CD4+ T cells and CD8+ T cells of splenocytes at day 7 after the third immunization. As shown in Figs. 5A~F, significantly higher percentages of PRRSV-specific CD4+IFN-γ+ T cells and CD8+IFN-γ+ T cells were detected in mice immunized with Yeast-GP5, both subcutaneously and orally. The expression levels of IFN-γ in CD4+ T cells and CD8+ T cells from mice vaccinated subcutaneously with Yeas-GP5 were 1.64- and 2.30-fold higher than those of the Yeast-vector group, respectively. For the oral immunization groups, the percentage of IFN-γ+CD4+ T cells and IFN-γ+CD8+ T cells were 2.95- and 4.0-fold higher than those of the control, respectively (Figs. 5C and F). These results suggest that Yeast-GP5 can stimulate both PRRSV-specific TH1 and CD8+ effector cell responses.
Various studies have shown that yeast is a desirable candidate for use as a vaccine platform [2,3]. For example, the nonpathogenic recombinant S. cerevisiae has been shown to stimulate both humoral and cell-mediated immune responses [3,6,27,30]. Similarly, K. lactis can be used as a delivery vehicle for subunit vaccines [4,9,32]. Here, we constructed recombinant K. lactis expressing HP-PRRSV GP5 (Yeast-GP5) and evaluated its immunogenicity in mice. We showed that the administration of Yeast-GP5 induced PRRSV-specific mucosal and cellular immune responses, suggesting that it could be an alternative vector for expression and presentation of PRRSV antigens.
Virus-specific antibody responses represent the humoral arm of adaptive immunity triggered during infection or vaccination. In this study, we demonstrated that mucosal immune responses in mice were elicited by oral administration of yeasts expressing HP-PRRSV GP5 protein. Intestinal washes of mice orally administered Yeast-GP5 showed higher levels of sIgA than those of mice that received Yeast-vector. The level of sIgA in vaginal washes of the Yeast-GP5 immunized group was also significantly higher than that in the control group, although the sIgA levels were lower than in small-intestinal washes. These findings are consistent with those of a previous report in which the authors showed that oral immunization induced substantial antibody responses in the small intestine, but were relatively inefficient at stimulating IgA antibody responses in female genital tract mucosa [16]. The intestine sIgA response generated by our subunit vaccine was comparable to that of transgenic plants expressing PRRSV antigen [17]. Overall, these findings indicate that yeast is an efficient mucosal delivery vehicle for stimulation of antigen-specific sIgA responses.
It is widely accepted that cell-mediated immunity is correlated with protective immunity against PRRSV infection. The frequency of PRRSV-specific IFN-γ secreting cells has been correlated with protection against reproductive failure in sows during outbreaks of PRRS in commercial herds [23,39]. In a previous study conducted by Zuckermann et al. [39], the authors found that the primary protective mechanism of a commercial modified-live PRRSV was due to the induced cellular immunity, which was characterized by production of virus-specific IFN-γ. In the present study, a strong T cell proliferation response was observed in mice subcutaneously injected with Yeast-GP5. This strong T cell proliferation was further supported by the detection of higher percentages of PRRSV-specific CD4+ and CD8+ IFN-γ-producing T cells in the spleen. The cellular immune responses induced by Yeast-GP5 were similar to those induced by recombinant Mycobacterium bovis BCG and recombinant adenovirus expressing PRRSV GP5 protein [5,35]. Recombinant adenovirus co-expressing GP3 and GP5 of highly pathogenic PRRSV fused with swine granulocyte-macrophage colony stimulating factor (rAd-GF35) could induce high levels of T lymphocyte proliferation and IFN-γ secretion in mice, and provide some protection against PRRSV challenge in pigs [35]. Our results suggest that Yeast-GP5 can confer cellular immunity to vaccinated animals. When mice were immunized orally, a higher percentage of IFN-γ positive CD4+ and CD8+ T cells was also observed in Yeast-GP5-immunized mice, although no significantly enhanced lymphocyte proliferation was detected. The strong PRRSV specific intestine mucosal sIgA response and higher percentage of IFN-γ-secreting cells suggest that oral vaccination with Yeast-GP5 could be a promising option. However, the stronger cellular immunity reflected by PRRSV-specific lymphocyte proliferation and virus-specific IFN-γ production in mice immunized subcutaneously with GP5-expressing K. lactis indicate that subcutaneous injection is more effective and feasible in practice.
We conducted a serum neutralization assay, which revealed only weak neutralization activity in serum samples from all groups. There are several possible reasons for the weak neutralization activity in serum samples. First, the inherent properties of the total recombinant yeast may play a role. Specifically, these yeast can efficiently mature and activate DCs to deliver heterologous antigens through non-classical cross-priming pathways, generating antigen-specific T-cells. Yeast expressing viral antigens can be degraded in proteasomes and endosomes, presented through MHC class I and MHC class II, leading to the recruitment and activation of antigen-specific CD4+ and CD8+ T cells. CD4+ T cells release immunostimulatory TH-1 type inflammatory cytokines, such as interleukin-2 (IL-2) and interferon-γ (IFN-γ), which further induce the activation and proliferation of CD8+ T cells [3,30]. Second, the dose of yeast applied and immunization route can also lead to weak neutralization. In our study, subcutaneous immunization with 2 × 108 Yeast-GP5 primarily induced T cell responses, while oral immunization with 5 × 108 Yeast-GP5 induced higher levels of intestinal mucosal sIgA and IFN-γ secretion. However, PRRSV-specific IgG induced by Yeast-GP5 in serum was relatively weak, regardless of the immunization routes we used here. A previous study showed that intramuscular injection of recombinant Hansenula polymorpha expressing hepatitis B virus surface antigen (yeast-HBsAg) induced both humoral and cellular immune responses in mice [7]. However, the production of HBsAg-specific IgG antibody was related to the number of yeast-HBsAg used for vaccination. When the number of yeast used was lower, almost no HBsAg-specific IgG antibodies were detected. The differences in the routes of immunization, expression levels of antigen in the yeast cells and numbers of applied yeast cells may account for the different results. In addition, since yeasts are eukaryotic, they are capable of modifying recombinant proteins according to a general eukaryotic scheme. However, several studies have shown that glycosylation of antigen played an important role in avoiding or minimizing virus-neutralizing Ab response via a N-glycan-shielding mechanism [11,36]. Inoculation of pigs with mutant viruses carrying mutations at N-glycosylation sites induced significantly higher levels of neutralizing antibodies against mutant and wild-type PRRSV [1]. Additionally, Jiang et al. [18] found that mice immunized with recombinant adenoviruses expressing GP5 with mutations at different glycosylation sites developed significantly enhanced neutralizing antibodies, suggesting that the loss of N-glycan residues in the ectodomain of GP5 enhances both the sensitivity of these viruses to neutralization and the immunogenicity of the neutralization epitopes. In the present study, no significant serum neutralization activity was detected, which may have been due to the glycosylation of GP5. Hence, further studies to modify the ORF5 gene by mutating the N-glycosylation sites, explore different immunization strategies, improve GP5 protein expression in K. lactis and optimize the immunization dose of Yeast-GP5 to enhance the immunogenicity of whole recombinant K. lactis expressing GP5 of PRRSV are warranted.
The measurement of cytokine production is essential to understanding adaptive immune response. IFN-γ are considered to be key cytokines in TH1 profiles and serve as indicators of many cell mediated responses, whereas IL-4 participates in TH2 polarization, and its secretion suggests a predominance of humoral responses. Therefore, we examined the levels of IL-4 and IFN-γ in CD4+ T cells and CD8+ T cells of splenocytes in immunized mice. Vaccination with Yeast-GP5 significantly induced IFN-γ (TH1) cytokine expression, but no significant difference in IL-4 (TH2) levels was observed in immunized groups. Interestingly, previous reports also showed that levels of IL-4 were below the detection limit following exposure to GI-5005 or yeast-CEA [6,14,27]. These results further demonstrated that the whole recombinant yeast had adjuvant potential and could effectively present antigen and generate antigen-specific T-cell immune responses [3].
Previous studies have shown that yeast is well-tolerated, and there have been no reports of serious adverse events upon its repeated administration [6,14]. In this study, no death, weight loss, or diarrhea was observed in Yeast-GP5 and Yeast-vector immunized mice during long term observation; however, it is still necessary to assess the safety of this recombinant yeast.
Figures and Tables
 | Fig. 1Construction and expression of the recombinant Kluyveromyces (K.) lactis. (A) Schematic diagram of Yeast-GP5 vaccine constructs. (B~D) Whole-cell polymerase chain reaction strategies with integration primers were designed to detect targeted integration of pKLAC1 into the K. lactis chromosome. Lane 1, Yeast-GP5; Lane 2, Yeast-vector; Lane M, DNA Marker. (B) 1.9-kb amplicon, single- or tandem-vector integration. (C) 2.3-kb amplicon, multicopy integration. (D) The GP5 gene of porcine reproductive and respiratory syndrome virus (PRRSV). (E) Analysis of GP5 expression in whole recombinant Yeast-GP5 in vitro by Western blotting. Lane 1, Yeast-vector as a negative control; Lane 2, Yeast-GP5-His as a positive control; Lane 3, Yeast-GP5. |
 | Fig. 2PRRSV-specific IgG and sIgA responses in immunized mice. (A) PRRSV-specific IgG in serum samples of mice immunized both subcutaneously and orally was analyzed by iELISA using a single dilution (1 : 250) on day 7 after the second immunization (day 14) and the third immunization (day 21). (B) Small-intestinal and vaginal washes of mice immunized orally were collected on day 7 after the third immunization to determine the PRRSV-specific sIgA. Levels of sIgA in secretions were detected by iELISA using a single dilution. The dilution of small-intestinal washes was 1 : 40, while that of vaginal washes was 1 : 20. Sc: subcutaneous. Data represent the mean ± standard deviation (SD). **p < 0.01. |
 | Fig. 3Lymphocyte proliferative responses in immunized mice. Splenocytes were isolated from vaccinated mice on day 7 after the final immunization and re-stimulated in vitro with purified HP-PRRSV antigen (10 µg/mL). Following 60 h of stimulation, MTT was added and the OD values were determined after 4 h of incubation. PMA + ionomycin as a positive control; bovine serum albumin (BSA, 2 µg/mL) as an irrelevant antigen control. Data are presented as the mean ± SD. **p < 0.01. |
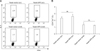 | Fig. 4PRRSV-specific IL-4 production in T cells. Splenocytes were isolated on day 7 after the final immunization and restimulated in vitro with purified HP-PRRSV antigen (10 µg/mL) for 6 h. The percentages of CD4+IL-4+ T cells were then analyzed by flow cytometry. (A) Scatter plot of one immunized mouse from each group. (B) Statistical assessment of IL-4-secreting cells among total CD4+ T cells in immunized mice. The vertical bars represent the mean ± SD. ns: not significant. |
 | Fig. 5PRRSV-specific IFN-γ production in T cells. Splenocytes were isolated and stimulated as in Fig. 4. (A and D) PE Rat IgG1 isotype control. (B and C) Summaries of percentages of IFN-γ+ CD4+ T cells in total CD4+ T cells. (B) Scatter plot of one immunized mouse from each group. (C) Statistical assessment. (E and F) Summaries of percentage of IFN-γ+ CD8+ T cells in total CD8+ T cells. (E) Scatter plot of one immunized mouse from each group. (F) Statistical assessment. Data are presented as the mean ± SD. *p < 0.05. |
Acknowledgments
This work was supported by the Faculty Starting Grant and State Key Laboratory of Agrobiotechnology (Grant No. 2010SKLAB06-1 and 2012SKLAB01-6), China Agricultural University, China.
References
1. Ansari IH, Kwon B, Osorio FA, Pattnaik AK. Influence of N-linked glycosylation of porcine reproductive and respiratory syndrome virus GP5 on virus infectivity, antigenicity, and ability to induce neutralizing antibodies. J Virol. 2006; 80:3994–4004.

2. Aouadi M, Tesz GJ, Nicoloro SM, Wang M, Chouinard M, Soto E, Ostroff GR, Czech MP. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature. 2009; 458:1180–1184.

3. Ardiani A, Higgins JP, Hodge JW. Vaccines based on whole recombinant Saccharomyces cerevisiae cells. FEMS Yeast Res. 2010; 10:1060–1069.
4. Arnold M, Durairaj V, Mundt E, Schulze K, Breunig KD, Behrens SE. Protective vaccination against infectious bursal disease virus with whole recombinant Kluyveromyces lactis yeast expressing the viral VP2 subunit. PLoS One. 2012; 7:e42870.
5. Bastos RG, Dellagostin OA, Barletta RG, Doster AR, Nelson E, Osorio FA. Construction and immunogenicity of recombinant Mycobacterium bovis BCG expressing GP5 and M protein of porcine reproductive respiratory syndrome virus. Vaccine. 2002; 21:21–29.

6. Bernstein MB, Chakraborty M, Wansley EK, Guo Z, Franzusoff A, Mostböck S, Sabzevari H, Schlom J, Hodge JW. Recombinant Saccharomyces cerevisiae (yeast-CEA) as a potent activator of murine dendritic cells. Vaccine. 2008; 26:509–521.

7. Bian G, Cheng Y, Wang Z, Hu Y, Zhang X, Wu M, Chen Z, Shi B, Sun S, Shen Y, Chen EJ, Yao X, Wen Y, Yuan Z. Whole recombinant Hansenula polymorpha expressing hepatitis B virus surface antigen (yeast-HBsAg) induces potent HBsAg-specific Th1 and Th2 immune responses. Vaccine. 2009; 28:187–194.

8. Blanquet S, Antonelli R, Laforet L, Denis S, Marol-Bonnin S, Alric M. Living recombinant Saccharomyces cerevisiae secreting proteins or peptides as a new drug delivery system in the gut. J Biotechnol. 2004; 110:37–49.

9. Böer E, Steinborn G, Kunze G, Gellissen G. Yeast expression platforms. Appl Microbiol Biotechnol. 2007; 77:513–523.

10. Bøtner A, Strandbygaard B, Sørensen KJ, Have P, Madsen KG, Madsen ES, Alexandersen S. Appearance of acute PRRS-like symptoms in sow herds after vaccination with a modified live PRRS vaccine. Vet Rec. 1997; 141:497–499.

11. Chen Z, Li K, Plagemann PG. Neuropathogenicity and sensitivity to antibody neutralization of lactate dehydrogenase-elevating virus are determined by polylactosaminoglycan chains on the primary envelope glycoprotein. Virology. 2000; 266:88–98.

12. Chia MY, Hsiao SH, Chan HT, Do YY, Huang PL, Chang HW, Tsai YC, Lin CM, Pang VF, Jeng CR. Immunogenicity of recombinant GP5 protein of porcine reproductive and respiratory syndrome virus expressed in tobacco plant. Vet Immunol Immunopathol. 2010; 135:234–242.

13. Du X, Zheng G, Jin H, Kang Y, Wang J, Xiao C, Zhang S, Zhao L, Chen A, Wang B. The adjuvant effects of co-stimulatory molecules on cellular and memory responses to HBsAg DNA vaccination. J Gene Med. 2007; 9:136–146.

14. Haller AA, Lauer GM, King TH, Kemmler C, Fiolkoski V, Lu Y, Bellgrau D, Rodell TC, Apelian D, Franzusoff A, Duke RC. Whole recombinant yeast-based immunotherapy induces potent T cell responses targeting HCV NS3 and Core proteins. Vaccine. 2007; 25:1452–1463.

15. Herre J, Gordon S, Brown GD. Dectin-1 and its role in the recognition of β-glucans by macrophages. Mol Immunol. 2004; 40:869–876.

17. Hu J, Ni Y, Dryman BA, Meng XJ, Zhang C. Immunogenicity study of plant-made oral subunit vaccine against porcine reproductive and respiratory syndrome virus (PRRSV). Vaccine. 2012; 30:2068–2074.

18. Jiang W, Jiang P, Wang X, Li Y, Wang X, Du Y. Influence of porcine reproductive and respiratory syndrome virus GP5 glycoprotein N-linked glycans on immune responses in mice. Virus Genes. 2007; 35:663–671.

19. Kimman TG, Cornelissen LA, Moormann RJ, Rebel JM, Stockhofe-Zurwieden N. Challenges for porcine reproductive and respiratory syndrome virus (PRRSV) vaccinology. Vaccine. 2009; 27:3704–3718.

21. Li B, Xiao S, Wang Y, Xu S, Jiang Y, Chen H, Fang L. Immunogenicity of the highly pathogenic porcine reproductive and respiratory syndrome virus GP5 protein encoded by a synthetic ORF5 gene. Vaccine. 2009; 27:1957–1963.

22. Li Y, Wang X, Bo K, Wang X, Tang B, Yang B, Jiang W, Jiang P. Emergence of a highly pathogenic porcine reproductive and respiratory syndrome virus in the Mid-Eastern region of China. Vet J. 2007; 174:577–584.

23. Lowe JE, Husmann R, Firkins LD, Zuckermann FA, Goldberg TL. Correlation of cell-mediated immunity against porcine reproductive and respiratory syndrome virus with protection against reproductive failure in sows during outbreaks of porcine reproductive and respiratory syndrome in commercial herds. J Am Vet Med Assoc. 2005; 226:1707–1711.

24. Music N, Gagnon CA. The role of porcine reproductive and respiratory syndrome (PRRS) virus structural and non-structural proteins in virus pathogenesis. Anim Health Res Rev. 2010; 11:135–163.

25. Ostrowski M, Galeota JA, Jar AM, Platt KB, Osorio FA, Lopez OJ. Identification of neutralizing and nonneutralizing epitopes in the porcine reproductive and respiratory syndrome virus GP5 ectodomain. J Virol. 2002; 76:4241–4250.

26. Qiu HJ, Tian ZJ, Tong GZ, Zhou YJ, Ni JQ, Luo YZ, Cai XH. Protective immunity induced by a recombinant pseudorabies virus expressing the GP5 of porcine reproductive and respiratory syndrome virus in piglets. Vet Immunol Immunopathol. 2005; 106:309–319.

27. Remondo C, Cereda V, Mostböck S, Sabzevari H, Franzusoff A, Schlom J, Tsang KY. Human dendritic cell maturation and activation by a heat-killed recombinant yeast (Saccharomyces cerevisiae) vector encoding carcinoembryonic antigen. Vaccine. 2009; 27:987–994.

28. Shen G, Jin N, Ma M, Jin K, Zheng M, Zhuang T, Lu H, Zhu G, Jin H, Jin M, Huo X, Qin X, Yin R, Li C, Li H, Li Y, Han Z, Chen Y, Jin M. Immune responses of pigs inoculated with a recombinant fowlpox virus coexpressing GP5/GP3 of porcine reproductive and respiratory syndrome virus and swine IL-18. Vaccine. 2007; 25:4193–4202.

29. Shin SJ, Shin SW, Kang ML, Lee DY, Yang MS, Jang YS, Yoo HS. Enhancement of protective immune responses by oral vaccination with Saccharomyces cerevisiae expressing recombinant Actinobacillus pleuropneumoniae ApxIA or ApxIIA in mice. J Vet Sci. 2007; 8:383–392.

30. Stubbs AC, Martin KS, Coeshott C, Skaates SV, Kuritzkes DR, Bellgrau D, Franzusoff A, Duke RC, Wilson CC. Whole recombinant yeast vaccine activates dendritic cells and elicits protective cell-mediated immunity. Nat Med. 2001; 7:625–629.

31. Van Breedam W, Van Gorp H, Zhang JQ, Crocker PR, Delputte PL, Nauwynck HJ. The M/GP(5) glycoprotein complex of porcine reproductive and respiratory syndrome virus binds the sialoadhesin receptor in a sialic acid-dependent manner. PLoS Pathog. 2010; 6:e1000730.

32. van Ooyen AJJ, Dekker P, Huang M, Olsthoorn MMA, Jacobs DI, Colussi PA, Taron CH. Heterologous protein production in the yeast Kluyveromyces lactis. FEMS Yeast Res. 2006; 6:381–392.
33. Van Reeth K. Pathogenesis and clinical aspects of a respiratory porcine reproductive and respiratory syndrome virus infection. Vet Microbiol. 1997; 55:223–230.

34. Vashisht K, Goldberg TL, Husmann RJ, Schnitzlein W, Zuckermann FA. Identification of immunodominant T-cell epitopes present in glycoprotein 5 of the North American genotype of porcine reproductive and respiratory syndrome virus. Vaccine. 2008; 26:4747–4753.

35. Wang X, Li J, Jiang P, Li Y, Zeshan B, Cao J, Wang X. GM-CSF fused with GP3 and GP5 of porcine reproductive and respiratory syndrome virus increased the immune responses and protective efficacy against virulent PRRSV challenge. Virus Res. 2009; 143:24–32.

36. Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. Antibody neutralization and escape by HIV-1. Nature. 2003; 422:307–312.

37. Zhou YJ, Hao XF, Tian ZJ, Tong GZ, Yoo D, An TQ, Zhou T, Li GX, Qiu HJ, Wei TC, Yuan XF. Highly virulent porcine reproductive and respiratory syndrome virus emerged in China. Transbound Emerg Dis. 2008; 55:152–164.

38. Zimmerman JJ, Yoon KJ, Wills RW, Swenson SL. General overview of PRRSV: a perspective from the United States. Vet Microbiol. 1997; 55:187–196.

39. Zuckermann FA, Garcia EA, Luque ID, Christopher-Hennings J, Doster A, Brito M, Osorio F. Assessment of the efficacy of commercial porcine reproductive and respiratory syndrome virus (PRRSV) vaccines based on measurement of serologic response, frequency of gamma-IFN-producing cells and virological parameters of protection upon challenge. Vet Microbiol. 2007; 123:69–85.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download