Introduction
Ultrasonography is commonly used for detecting pneumoperitoneum in humans [7]. Pneumoperitoneum is characterized by the sonographic appearance of linear enhancement and thickening of the enhanced peritoneal stripe sign (EPSS) associated with either dirty shadowing or multiple reflection artifacts at the air-soft tissue interface between the nondependent abdominal wall and intra-abdominal structures such as the liver, stomach, omentum, or intestine [6,8]. Ultrasonographic detection of free intra-peritoneal air using the EPSS is possible for making a primary diagnosis in the emergency room [5]. Prompt identification of pathological conditions reduces the delay in management of seriously injured patients and reduces secondary complications.
In our previous study, we evaluated the ultrasonographic detection of pneumoperitoneum in beagles subjected to graded injections of intraperitoneal air [2]. The minimal volume of free air that could be detected via ultrasonography was 0.1 mL. The purpose of the present investigation was to determine the feasibility and accuracy of estimating the optimal minimum amount of abdominal free gas for inducing the EPSS for ultrasonographic examination with a blinded operator in a large population of dogs.
Materials and Methods
Healthy adult beagles (128 males and 154 females) weighing from 6.6 to 13.8 kg and between 2 ~ 3 years old were used for the study. Animals care and experiments were carried out according to the "Guide for the Care and Use of Laboratory Animals" from Gyeongsang National University, Korea. The beagles were judged to be in good to excellent health based upon physical examination, hemogram results, serum chemistry, abdominal radiographs, and abdominal ultrasonography findings. In particular, preliminary data including that from complete ultrasonographic examination with emphasis placed on identifying cases of pneumoperitoneum were utilized.
The beagles were divided into three groups. Group A included 94 dogs (40 males and 54 females). Fifty eight dogs of this group received 0.1 mL of air injected into the peritoneal cavity while the rest of them did not receive. Group B consisted of 94 dogs (46 males and 48 females). Fifty eight dogs of group B received 0.2 mL of air injected into the peritoneal cavity. The rest of them were not injected intraperitoneal air. In group C, 94 dogs (42 males and 52 females) were allocated. Some (58 dogs) of them received 0.5 mL of intraperitoneal air while 36 dogs did not receive. The beagles were anesthetized with propofol (Provive 1%; Myungmoon Pharm, Korea) for intubation. Anesthesia was maintained with 1.9% to 2.2% isoflurane (Ifran; Hana Pharm, Korea). Using ultrasonographic guidance, a 20-gauge angiocatheter (Angiocath Plus; BD Medical, Singapore) was placed into the peritoneal cavity along the midline at the level of the umbilical region for proper air injection. Next, 0.1, 0.2, and 0.5 mL of air were injected into the cranial aspect of the intraperitoneal space near the umbilicus. In randomly selected dogs of each group, the angiocatheters were just removed after placed into the subcutaneous tissue without air injection for the negative control. Prior to ultrasonographic examination, abdominal massage was performed for 5 min to properly distribute the air with the dog in a standing position.
An ultrasound machine (Xario SSA-660A; Toshiba, Japan) was used with a 12.0 MHz linear transducer. The abdomen was prepared by clipping the hair over the entire ventral aspect of the abdomen. Coupling gel (Acoustic Gel; Seoungwon, Korea) was applied to the skin. The animals were scanned while in a dorsal recumbent position. All ultrasonographic procedures were performed entirely by the same examiner (SY Kim) who had proficient experience with ultrasonography and was blinded to group assignments and the presence of intraperitoneal air. Peritoneal free gas was identified as the EPSS during the ultrasonographic examinations (Fig. 1). A systematic search for the EPSS was carried out in all regions, and presence of this feature was noted. Magnified images were used in most cases with appropriate adjustment of the gain, focal point, and other settings.
To determine the accuracy of ultrasonographic diagnosis of pneumoperitoneum using the EPSS, we defined true-positive (TP), true-negative (TN), false-positive (FP), and false-negative (FN) findings as follows. A TP result was defined as a case in which the EPSS was identified as air was injected into the peritoneal cavity. A TN finding was defined as a case in which the EPSS was not identified as air was injected into the peritoneal cavity. FP indicated that the EPSS was identified when air was not injected into the peritoneal cavity. Finally, a FN indicated that the EPSS not identified as air was injected into the peritoneal cavity. Sensitivity, specificity, accuracy, likelihood ratio, and positive and negative predictive values of ultrasonography for detecting the EPSS were determined separately for each group using the following formulae:
Results
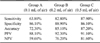
No association between age, gender, or body weight and the diagnostic accuracy of ultrasonography was observed. Ultrasonographic examinations of all animals were successfully completed. There were 37 (39%) TP findings, 31 (33%) TN results, five (5%) FP findings, and 21 (23%) FN cases in group A. In group B, there were 48 (51%) TP results, four (4%) TN cases, 32 (34%) FP results, and 10 (11%) FN cases. Group C included 51 (54%) TP results, five (5%) TN findings, 31 (33%) FP cases, and seven (8%) FN results.
Sensitivity, specificity, accuracy, positive predictive values, and negative predictive values for each group are presented in Table 1. The levels of accuracy for groups A, B, and C were 72.3%, 85.1%, and 87.2%, respectively. Positive predictive values for groups A, B, and C were 88.1%, 92.3%, and 91.1%, respectively. Accuracy and positive predictive values for group A were lower than those of the other groups. Additionally, the positive (negative) likelihood ratios for identifying the EPSS were 4.59 (0.42), 7.45 (0.19), and 6.33 (0.14) for groups A, B, and C, respectively.
Discussion
Since first use of the EPSS for diagnosing pneumoperitoneum in humans was initially reported [8], a few studies have explored the application of the EPSS for diagnosing pneumoperitoneum in other species [1,2]. Though pneumoperitoneum is not always detectable following viscus perforation, identification of pneumoperitoneum in a human presenting with acute abdominal pain is considered to be one of the most significant signs in medicine, and over 90% of cases will require emergency surgery [4]. Therefore, it is important to confirm that the EPSS is a reliable and specific sonographic characteristic for identifying cases of pneumoperitoneum.
The clinical implication of a false-positive diagnosis of pneumoperitoneum could lead to additional unnecessary examinations or invasive procedures [1,3]. Therefore, in this study we tried to differentiate the EPSS from potential artifacts including overlying rib reflection, ring-down artifacts from the adjacent air-filled lung, and interposition of the colonic gas cranial to the liver. Despite our efforts to accurately identify the EPSS, false-positive results were obtained for some experimental groups. Group A had a particularly high rate of false-positives. Misidentified EPSS-like signs were usually observed near the linea alba. This may have been because the thickened focal linea alba has an appearance similar to focal enhancement and apparent thickening of the peritoneal stripe induced by the EPSS. To the best of our knowledge, no report describing this drawback has been published, but our results indicate that it can sometimes be difficult to differentiate the EPSS from the thickened focal linea alba.
Sensitivity, specificity, accuracy, and positive and negative predictive values for groups B and C were sufficiently high to indicate that the EPSS is a useful and specific sign for detecting peritoneal free air in dogs. Based on the higher positive likelihood ratio of group B, the minimum volume of air that could be reliably and consistently detected was 0.2 mL. The EPSS could therefore verify cases of pneumoperitoneum if more than 0.2 mL of intra-abdominal free gas is present.
This study has some limitations worth noting. The investigation was performed by a single blinded operator and carried out on experiment animals that received artificial injections of peritoneal air rather than actual patients. It is possible that the ultrasonographic findings of the abdomen in patients with naturally occurring cases of pneumoperitoneum are different from those of the experimental canines. In actual patients, intercurrent complications such as peritonitis and gastrointestinal wall thickness have certainly been identified. Furthermore, this study was not performed to assess the reproducibility of the smallest estimated amount of abdominal free gas that could be detected by ultrasonography. Additional case studies involving ultrasonographic examinations of the abdomen, particularly those of patients with intestinal perforations, will therefore be necessary to evaluate the clinical validation of ultrasonographic EPSS detection.
In conclusion, the minimal amount of abdominal free gas that could be identified by ultrasonography for diagnostic purposes was 0.2 mL. This result, when combined with further study which will carry out in experimentally induced canine pnemoperitoneum,, can be utilized as an important criterion for diagnosing pneumoperitoneum in the future. Additionally, the thickened focal linea alba may mimic EPSS which cause misdiagnosis of pneumoperitoneum during ultrasonographic examination.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download