Abstract
Type 1 diabetes is a common metabolic disorder accompanied by increased blood glucose levels along with glucocorticoid and cognitive deficits. The disease is also thought to be associated with environmental changes in brain and constantly induces oxidative stress in patients. Therefore, glucocorticoid-mediated negative feedback mechanisms involving the glucocorticoid receptor (GR) binding site are very important to understand the development of this disease. Many researchers have used streptozotocin (STZ)-treated diabetic animals to study changes in GR expression in the brain. However, few scientists have evaluated the hyperglycemic period following STZ exposure. In the present study, we found GR expression in the hippocampus varied based on the period after STZ administration for up to 4 weeks. We performed immunohistochemistry and Western blotting to validate the sequential alterations of GR expression in the hippocampus of STZ-treated type 1 diabetic rats. GR protein expression increased significantly until week 3 but decreased at week 4 following STZ administration. GR expression after 70 mg/kg STZ administration was highest at 3 weeks post-treatment and decreased thereafter. Although STZ-induced increase in GR expression in diabetic animals has been described, our data indicate that researchers should consider the sequential GR expression changes during the hyperglycemic period following STZ exposure.
The hippocampus is an important integration center for learning and memory [14], and one of the major target areas of glucocorticoids (GCs) in the central nervous system [14,19]. GCs are also essential for maintaining neurons and are hyperglycemic agents that promote diabetes [18,29]. Furthermore, GCs in the blood easily cross the blood-brain barrier and bind to the GC receptor (GR) in the brain [13,27]. GC effects are mediated by ligand-dependent activation of the GR, which is detected in almost every type of cell [21]. Mineralocorticoid receptors (MRs) in the brain are involved in the regulation of stress hormone secretion and complex processes such as ones that control emotions, memory, and sleep [12]. MRs also regulate salt balance and water homeostasis by directly stimulating the expression of specific ionic transporters that are active in the cellular membrane [30]. Although many reports about MR regulation have been published, the exact mechanisms involved in the actions of this receptor remain unknown [2,6]. However, the GR plays critical roles in neuronal regeneration and death as well as learning behaviors, memory, and adaptation within the brain [25,32]. We have previously examined the GR and its involvement in gluconeogenesis along with glucose transport in cases of diabetes [11,16,20].
Chronic stress and prolonged exposure to GCs can damage the hippocampus and induce hippocampal degeneration [36]. The importance of GR-mediated effects is observed with metabolic disorders, particularly diabetes [1,16,26,28,32]. Diabetes is closely associated with increased basal hypothalamo-pituitary-adrenal activity and impaired stress responsiveness [7,8,31]. In addition, activation of the GR was found to modulate the system associated with memory, behavior, anxiety, and fear [27,31,36]. Several reports have been published about GR expression in the hippocampus under diabetic conditions [4,14,15,16,23,28]. However, no study has evaluated GR expression in the hippocampus of animal models with streptozotocin (STZ)-induced type 1 diabetes as it relates to disease development stages. Many researchers have used STZ to induce type 1 diabetes; however, the time and duration of STZ treatment has varied among studies [15,22,24,28,35]. Yi et al. [34] reported that chronological changes in superoxide dismutase activity and inflammatory response in the hippocampus occur 2~4 weeks after STZ administration. Thus, we hypothesized that GR expression in the hippocampus is different based on the time after STZ treatment even though STZ-induced type 1 diabetes develops within a few days after STZ administration. In this study, we therefore assessed GR immunoreactivity and protein level changes in the hippocampus of STZ-treated type 1 diabetic rats 2~4 weeks after STZ injection.
Male Wistar rats (n = 40, 7 weeks old) were purchased from OrientBio (Korea). The animals were housed at 23℃ and 60% relative humidity with a 12-h light/dark cycle, and had free access to food and water. The rats were fed a Purina 5008 rodent diet (7.5% fat) as recommended by the manufacturer (Ralston Purina, USA). Animal handling and care procedures adhered to currently accepted guidelines (National Institutes of Health [NIH] Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85-23, 1985, revised 1996) and this study was approved by the Institutional Animal Care and Use Committee of Seoul National University, College of Veterinary Medicine (Seoul, Korea). All experiments were conducted in a manner that minimized the number of animals used and any suffering caused by the procedures.
Type 1 diabetes was induced with a single intra-peritoneal (i.p.) injection of 70 mg/kg/5 mL STZ (Sigma-Aldrich, USA) diluted with 0.1 M sodium citrate buffer (pH 4.3) prior to use. Fasting blood glucose levels were monitored after 72 h, and rats with blood glucose concentrations > 145 mg/dL were divided into three groups. Group 1 was assessed 2 weeks after the STZ injection (STZ2w), group 2 was monitored after 3 weeks (STZ3w), and group 3 was evaluated 4 weeks after the STZ treatment (STZ4w). Control rats received 0.1 M sodium citrate buffer (pH 4.3) in a single i.p. injection as vehicle.
Blood was collected weekly from tail veins, and blood glucose levels were measured using a validated one-touch basic glucose measurement system (SureStep blood glucose meter; LifeScan, USA). Body weight was also measured weekly from baseline. Food intake and water consumption of the control and STZ-treated groups were recorded twice a week at 09:00.
Plasma (50 µL) was added to 5 mL of methylene chloride and incubated at room temperature for 10 min. After filtration through cheesecloth, the mixture was combined with 2.5 mL of fluorescence reagent (7 : 3, sulfuric acid : absolute ethanol), vortexed vigorously, and incubated for 30 min at room temperature. After centrifugation, absorbance of the lower layer was measured using a spectrophotometer (excitation wavelength, 475 nm; emission wavelength, 530 nm, UV-1601; Shimadzu, Japan).
Five animals from each group (control, STZ2w, STZ3w, and STZ4w) were anesthetized with sodium pentobarbital at designated times and perfused transcardially with 0.1 M phosphate-buffered saline (PBS; pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The brains were removed and post-fixed in the same fixative [4% paraformaldehyde in Phosphate buffer (pH7.4)] for 6 h at room temperature. The brain tissues were then cryoprotected by infusion with 30% sucrose overnight, and the frozen tissues were serially sectioned (30-µm thickness) in the coronal plane using a cryostat microtome (Thermo Fisher Scientific, Germany) at -20℃. The sections were then placed into 24-well plates containing PBS.
Immunohistochemistry was performed under the same conditions for all groups. The brain sections were treated with 0.3% hydrogen peroxide in PBS for 30 min and then with 10% normal goat serum (Vector Laboratories, USA) in 0.05 M PBS for 30 min at room temperature. The sections were then incubated with rabbit anti-GR antibody (1 : 1,000 dilution, clone M-20; Santa Cruz Biotechnology, USA) overnight at room temperature, and subsequently exposed to biotinylated goat anti-rabbit IgG and streptavidin peroxidase complex (1 : 200 dilution; Vector Laboratories) at room temperature. Antibody binding was visualized by staining with 3,3'-diaminobenzidine in 0.1 M Tris-HCl buffer (pH 7.2). The sections were mounted on gelatin-coated slides and dehydrated. Negative control samples were incubated with pre-immune serum instead of primary antibody to measure immunostaining specificity.
Five animals each from the control, STZ2w, STZ3w, and STZ4w groups were sacrificed, and Western blot analysis was performed to confirm changes in GR levels in the hippocampus at each time point. The brains were serially and transversely sectioned (400-µm thickness) with a vibratome (Leica, USA), and the hippocampi were removed using a surgical blade. The hippocampal tissues were homogenized in 50 mM PBS (pH 7.4) containing 0.1 mM ethylene glycol bis (2-aminoethyl ether)-N,N,N',N' (pH 8.0), 0.2% Nonidet P-40, 10 mM ethylendiamine tetra-acetic acid (pH 8.0), 15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, 50 mM NaF, 150 mM NaCl, 2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and 1 mM dithiothreitol (DTT). After centrifugation, protein levels in supernatants were measured using a Micro BCA Protein Assay kit (Pierce chemical, USA) with bovine serum albumin (Sigma-aldrich) as the standard. Briefly, aliquots containing 80 µg of total protein were boiled in loading buffer containing 150 mM Tris (pH 6.8), 3 mM DTT, 6% sodium dodecyl sulfate, 0.3% bromophenol blue, and 30% glycerol. Aliquots (30 µg) of the protein samples were then loaded onto 10% polyacrylamide gels, and the proteins were transferred onto nitrocellulose membranes (Pall Corporation, USA) after electrophoresis. The membranes were incubated with 5% non-fat dry milk in PBS containing 0.1% Tween 20 for 45 min to reduce non-specific binding and then incubated with rabbit anti-GR (1 : 2,000 dilution; Santa Cruz Biotechnology) overnight at 4oC followed by peroxidase-conjugated goat anti-rabbit IgG (1 : 5,000; Sigma-aldrich) for 2 h at room temperature. Antibody binding was visualized by enhanced chemiluminescence using a kit (Pierce chemical, USA).
All measurements were performed and analyzed blindly by two researchers per experiment to ensure objectivity. Ten sections per animal were selected to quantitate GR-immunoreactive cells in the hippocampus. The counts were performed using an image analyzing system equipped with a computer-based CCD camera (Optimas 6.5; Cybermetrics, USA). The mean number of GR-immunopositive cells per hippocampus section was determined. The staining intensity of GR-immunoreactive structures was evaluated based on optical density (OD) measured by transforming mean gray levels using the formula: OD = log (256/mean gray level). Background OD levels were subtracted from those of areas adjacent to the regions of interest. OD ratios were then expressed as percentages (relative optical density; ROD) using Adobe Photoshop version CS2 (Adobe Systems, USA) and then analyzed using NIH Image 1.59 software. In addition, the Western blots were scanned, and band intensity was quantified using Scion Image software (Scion Corporation, USA), which was used to determine ROD. The ROD ratio was calibrated as a percentage. Data are presented the mean ± standard error (SE). Differences between mean values were analyzed using a two-way analysis of variance followed by Tukey's test and Duncan's new multiple range method. A p value < 0.05 was considered significant.
Mean body weight of the STZ-treated rats decreased over the 4-week experimental period whereas that of the controls increased (Fig. 1A). STZ-treated rats had blood glucose levels that were more than 4-times greater than those of the control animals (Fig. 1B). Additionally, the STZ-exposed animals consumed significantly more food (Fig. 1C) and water (Fig. 1D) than the controls.
Plasma corticosterone levels of the STZ2w, STZ3w, and STZ4w rats were significantly higher than those of controls. However, these levels were reduced in the STZ3w group compared to those of the STZ2w and STZ4w animals (Fig. 2).
GR-specific immunoreactivity was detected in the hippocampus (CA1 and dentate gyrus; DG) but rarely found in the CA3 hippocampal area. Immunoreactive cell counts in the CA1 of all groups were not significantly different except in the DG of the STZ3w and STZ4w groups (Fig. 3E). However, the intensity of the CA1 cells in each group differed over time (Figs. 3A1, B1, C1, and D1) although the number of immunopositive cells in the CA1 did not vary (Fig. 3E). The numbers and intensities of the immunoreactive cells in the DG were significantly different when comparing the STZ3w group and control animals (Figs. 3E and F). These immunohistochemistry assay results only indicated the general trends in the hippocampal CA1 region and did not reflect quantitative changes. However, the intensity and number of immunopositive cells in the DG region differed among the STZ-treated groups.
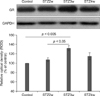
GR protein expression in the hippocampus concurred with the observed changes in immunohistochemical staining intensity (Fig. 4). GR protein levels significantly increased in the STZ3w group (1.32-times higher than that in the control; p < 0.05) and were reduced in the STZ4w animals (1.14-times higher than that in the control). Overall, GR expression levels tended to increase relative to the control.
Many studies about GR expression in cases of type 1 diabetes have been conducted [5,10,16,17,24,28]. A chronic excess of GCs can evoke similar to neuropathological signals linked to major targets in the brain [15,24,32]. Excessive stimulation of the GR by STZ can result in compromised neuronal viability and cognitive performance associated with hippocampal function in diabetic animals. Blocking the GR during early type 1 diabetes development attenuates the morphological signs of hippocampal disorders and rescues diabetic animals from cognitive deficits [28]. Therefore, GR expression during this early phase is critically important for preventing STZ-induced hippocampal dysfunction. In the present investigation, we measured GR immunoreactivity and observed expressional differences in the hippocampus of STZ-treated type 1 diabetic rats until 4 weeks after STZ administration. GR immunoreactivity was observed mainly in the CA1 and DG regions of the hippocampus but not in the CA3 region. GR-immunopositive cell counts were significantly different in the DG region but not the CA1 region. However, GR-specific immunostaining intensity in both regions appeared different. We thus measured the signals using an image analyzer (Fig. 3C). The Western blot assay showed that GR protein expression increased continuously and sequentially in the STZ2w and STZ3 groups compared to the control and then decreased thereafter in STZ4w animals (Fig. 4). However, expression levels remained high although the increase was not significant.
Ranhotra and Sharma [24] examined GR expression based on tissue- and age-specific variations in STZ-treated diabetic mice. Revsin et al. [28] reported that excessive GR stimulation compromises neuronal viability and cognitive performance associated with hippocampal function in STZ-treated type 1 diabetic mice. Grillo et al. [14] examined diabetes-mediated plasticity in the hippocampus of STZ-exposed type 1 diabetic rats. However, the time points at which these research groups chose to sacrifice the animals were not the same. Ranhotra and Sharma showed differences in the kidney and liver at two time points (15 and 120 days post-STZ injection); however, they did not show their data collected between these time points [24]. Thus, this group may have ignored changes in body conditions caused by STZ administration. Revsin et al. [28] evaluated changes 6 and 11 days after STZ administration. Grillo et al. [14] examined the animals 1 (short-term diabetic animals) and 5 weeks (long-term diabetic animals) after the development of hyperglycemia. Researchers have considered the effects of short- and long-term STZ exposure times. Nevertheless, they have ignored the effects of STZ injection that could develop over time. GR expression is generally thought to increase in many brain regions under diabetic conditions due to dysregulatory effects in the body regardless of the type of diabetes [14,16,24,32,33]. STZ has been used to induce type 1 diabetes in rodents but researchers have not fully examined the effects of STZ exposure time or dose following administration. If blood glucose levels are reasonably high, many researchers conduct their experiments without considering a timed exposure [14,24,28]. Some discrepancies in the results have therefore occurred [14,24,28]. However, some groups have considered several possibilities when interpreting their experimental results [22,34]. In particular, the possibility that the hippocampal microenvironment could be altered after systemic STZ exposure has been proposed [34], and several microenvironmenal changes in the hippocampus were found 3 weeks after STZ injection. Therefore, we attempted to identify changes in hippocampal GR expression over time (STZ2w, STZ3w, and STZ4w). Yi et al. [34] examined chronological changes in superoxide dismutase 1 (SOD1) activity and inflammatory responses in the hippocampus of STZ-treated type 1 diabetic animals. For this study, assessments were made at three time points (2, 3, and 4 weeks) following STZ administration to rats. Several markers related to oxidative stress and inflammatory responses in the hippocampus were significantly enhanced or reduced 3 weeks after STZ injection versus the controls. Therefore, the 3-week time point was thought to be a very critical period for hippocampal changes following systemic STZ administration. However, it is unclear whether this is attributed to microenvironmental changes due to the length of time after STZ injection. Further study is necessary for clarification.
Basal plasma corticosterone levels are significantly elevated in diabetic rats [3,9]. Hwang et al. [15] reported that GR expression levels in the hippocampus simultaneously increase with elevated plasma corticosterone levels in cases of type 2 diabetes. In the present study, plasma corticosterone levels in the STZ2w, STZ3w, and STZ4w groups were significantly higher than those in the control. However, the plasma corticosterone levels in the STZ3w group were decreased compared to those in the STZ2w and STZ4w groups although they were significantly higher than the levels in the control animals. Since GR expression in the STZ3w group was highest among the time points, physiological factors that influence the relationship between corticosterone and GR expression in the hippocampus should be further investigated. However, we found that GR expression changes were not affected based only on alterations in plasma corticosterone levels if these levels were significantly high. The GR protein levels in the STZ3w to STZ4w groups were not perfectly matched (Figs. 3 and 4), and GR expression in the hippocampus decreased in the STZ3w rats. It should be noted that immunohistochemistry results to calculate quantity are not accurate alone. Therefore, results may not be acceptable when these data are the only evidence for differences in protein levels. The results in Fig. 3. show changes in expression over time. Sections of the hippocampal area used for immunohistochemistry were only some of the ones that were collected. STZ4w GR expression in the hippocampus tended to decrease compared to that of the STZ3w group (Fig. 3). No difference was observed between STZ3w and STZ4w rats (Figs. 3 and 4).
In conclusion, GR expression increased progressively until 3 weeks after STZ injection and decreased thereafter. This may not be a direct effect of corticosterone because levels of this factor remained high after 4 weeks. In addition, we found that the largest difference in hippocampal GR expression after STZ administration compared to that of the control occurred in the STZ3w group. A number of reports have described GR expression in STZ-treated type 1 diabetes animal models [15,24,28,32], However, the times at which the GR is activated post-STZ administration have not been widely examined. We thus measured GR protein expression at three time points following STZ treatment, and found that GR expression was altered over time after STZ exposure. Therefore, we suggest that researchers should consider the time at which animal sacrifice is conducted (the experimental end-point) after STZ treatment to obtain accurate results when measuring GR expression in the hippocampus of STZ-treated animals.
Figures and Tables
Fig. 1
Physiological data for the control and STZ-treated rats. Body weights of the vehicle group exponentially increased while those of the STZ-treated rats slowly decreased over the 4-week study period (A). Blood glucose levels at baseline and 1, 2, 3, and 4 weeks post-STZ treatment were significantly different from those of the control. Blood glucose levels continuously increased in STZ-treated animals from 385 mg/dL at 1 week (B). Food intake and water consumption also noticeably increased from 1 week post-STZ treatment (C and D; *p < 0.005). Results are expressed as the mean ± SE.

Fig. 2
Plasma corticosterone levels in STZ-treated rats. Plasma corticosterone levels at 2-, 3-, and 4-weeks post-STZ treatment (STZ2w, STZ3w, and STZ4w, respectively) were significantly different from those of the control (n = 5 per group, *p < 0.001 and **p < 0.05). Plasma corticosterone levels were reduced in the STZ3w group; however, the levels were significantly high than that of the control. Results are expressed as the mean ± SE.
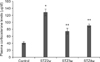
Fig. 3
Immunoreactivities specific for the glucocorticoid receptor (GR) in the hippocampus (CA1 and dentate gyrus; DG) of control and STZ-treated rats at 2, 3, and 4 weeks after STZ administration. GR-immunopositive cell counts (E) and relative optical densities (ROD) expressed as % values of GR (F) relative to that of the control animals in the CA1 and DG are presented in the bar graphs. The fields showing GR-specific immunostained cells in the CA1 (C1) and DG (C2) are magnified. The GR-immunopositive cell counts for the CA1 of each group were not significantly different. However, those in the DG from STZ3w to STZ4w were significantly higher compared to the numbers found in the control (E; *p < 0.05). The intensities of GR-specific immunostained cells were significantly increased from STZ3w to STZ4w in the CA1 and DG regions compared to the control (F; *p < 0.05 and **p < 0.001). Results are expressed as the mean ± SE. Scale bars = 100 µm.
Fig. 4
Western blot analysis of GR expression in the hippocampus region of the control and STZ-treated rats 2, 3, and 4 weeks (STZ2w, STZ3w and STZ4w, respectively) after STZ exposure. ROD values are expressed as percentages versus GR protein levels observed in the control (n = 5 per group). The values were normalized to those of GAPDH. Results are expressed as the mean ± SE.
References
1. Ahima R, Krozowski Z, Harlan R. Type I corticosteroid receptor-like immunoreactivity in the rat CNS: distribution and regulation by corticosteroids. J Comp Neurol. 1991; 313:522–538.

2. Alnemri ES, Maksymowych AB, Robertson NM, Litwack G. Overexpression and characterization of the human mineralocorticoid receptor. J Biol Chem. 1991; 266:18072–18081.

3. Barber M, Kasturi BS, Austin ME, Patel KP, MohanKumar SMJ, MohanKumar PS. Diabetes-induced neuroendocrine changes in rats: role of brain monoamines, insulin and leptin. Brain Res. 2003; 964:128–135.

4. Beauquis J, Homo-Delarche F, Giroix MH, Ehses J, Coulaud J, Roig P, Portha B, De Nicola AF, Saravia F. Hippocampal neurovascular and hypothalamic-pituitary-adrenal axis alterations in spontaneously type 2 diabetic GK rats. Exp Neurol. 2010; 222:125–134.

5. Bitar MS. Co-administration of etomoxir and RU-486 mitigates insulin resistance in hepatic and muscular tissues of STZ-induced diabetic rats. Horm Metab Res. 2001; 33:577–584.

6. Chan O, Chan S, Inouye K, Vranic M, Matthews SG. Molecular regulation of the hypothalamo-pituitary-adrenal axis in streptozotocin-induced diabetes: effects of insulin treatment. Endocrinology. 2001; 142:4872–4879.

7. Chan O, Inouye K, Vranic M, Matthews SG. Hyperactivation of the hypothalamo-pituitary-adrenocortical axis in streptozotocin-diabetes is associated with reduced stress responsiveness and decreased pituitary and adrenal sensitivity. Endocrinology. 2002; 143:1761–1768.

8. Chan O, Inouye K, Akirav E, Park E, Riddell MC, Vranic M, Matthews SG. Insulin alone increases hypothalamo-pituitary-adrenal activity, and diabetes lowers peak stress responses. Endocrinology. 2005; 146:1382–1390.

9. Chan O, Inouye K, Akirav EM, Park E, Riddell MC, Matthews SG, Vranic M. Hyperglycemia does not increase basal hypothalamo-pituitary-adrenal activity in diabetes but it does impair the HPA response to insulin-induced hypoglycemia. Am J Physiol Regul Integr Comp Physiol. 2005; 289:R235–R246.

10. De Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998; 19:269–301.

11. Dimitriadis GD, Raptis SA. Thyroid hormone excess and glucose intolerance. Exp Clin Endocrinol Diabetes. 2001; 109:Suppl 2. S225–S239.

12. Erickson K, Drevets W, Schulkin J. Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neurosci Biobehav Rev. 2003; 27:233–246.

13. Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-β and tau pathology in a mouse model of Alzheimer's disease. J Neurosci. 2006; 26:9047–9056.

14. Grillo CA, Piroli GG, Wood GE, Reznikov LR, McEwen BS, Reagan LP. Immunocytochemical analysis of synaptic proteins provides new insights into diabetes-mediated plasticity in the rat hippocampus. Neuroscience. 2005; 136:477–486.

15. Hwang IK, Yi SS, Yoo KY, Park OK, Yan B, Song W, Won MH, Yoon YS, Seong JK. Effect of treadmill exercise on blood glucose, serum corticosterone levels and glucocorticoid receptor immunoreactivity in the hippocampus in chronic diabetic rats. Neurochem Res. 2011; 36:281–287.

16. Jöhren O, Dendorfer A, Dominiak P, Raasch W. Gene expression of mineralocorticoid and glucocorticoid receptors in the limbic system is related to type-2 like diabetes in leptin-resistant rats. Brain Res. 2007; 1184:160–167.

17. McEwen BS, De Kloet ER, Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986; 66:1121–1188.

20. Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest. 1998; 101:2174–2181.

21. Opherk C, Tronche F, Kellendonk C, Kohlmüller D, Schulze A, Schmid W, Schütz G. Inactivation of the glucocorticoid receptor in hepatocytes leads to fasting hypoglycemia and ameliorates hyperglycemia in streptozotocin-induced diabetes mellitus. Mol Endocrinol. 2004; 18:1346–1353.

22. Paskitti ME, McCreary BJ, Herman JP. Stress regulation of adrenocorticosteroid receptor gene transcription and mRNA expression in rat hippocampus: time-course analysis. Brain Res Mol Brain Res. 2000; 80:142–152.

23. Petersson M, Uvnäs-Moberg K. Systemic oxytocin treatment modulates glucocorticoid and mineralocorticoid receptor mRNA in the rat hippocampus. Neurosci Lett. 2003; 343:97–100.

24. Ranhotra HS, Sharma R. Streptozotocin-induced diabetes and glucocorticoid receptor regulation: tissue- and age-specific variation. Mech Ageing Dev. 2000; 119:15–24.

25. Rashid S, Lewis GF. The mechanisms of differential glucocorticoid and mineralocorticoid action in the brain and peripheral tissues. Clin Biochem. 2005; 38:401–409.

27. Reul JMHM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985; 117:2505–2511.

28. Revsin Y, Rekers NV, Louwe MC, Saravia FE, De Nicola AF, de Kloet ER, Oitzl MS. Glucocorticoid receptor blockade normalizes hippocampal alterations and cognitive impairment in streptozotocin-induced type 1 diabetes mice. Neuropsychopharmacology. 2009; 34:747–758.

29. Thompson A, Arany EJ, Hill DJ, Yang K. Glucocorticoid receptor expression is altered in pancreatic β cells of the non-obese diabetic mouse during postnatal development. Metabolism. 2002; 51:765–768.

30. Viengchareun S, Penfornis P, Zennaro MC, Lombès M. Mineralocorticoid and glucocorticoid receptors inhibit UCP expression and function in brown adipocytes. Am J Physiol Endocrinol Metab. 2001; 280:E640–E649.

31. Yi SS, Hwang IK, Kim YN, Kim IY, Pak SI, Lee IS, Seong JK, Yoon YS. Enhanced expressions of arginine vasopressin (Avp) in the hypothalamic paraventricular and supraoptic nuclei of type 2 diabetic rats. Neurochem Res. 2008; 33:833–841.

32. Yi SS, Hwang IK, Chun MS, Kim YN, Kim IY, Lee IS, Seong JK, Yoon YS. Glucocorticoid receptor changes associate with age in the paraventricular nucleus of type II diabetic rat model. Neurochem Res. 2009; 34:851–858.

33. Yi SS, Hwang IK, Shin JH, Choi JH, Lee CH, Kim IY, Kim YN, Won MH, Park IS, Seong JK, Yoon YS. Regulatory mechanism of hypothalamo-pituitary-adrenal (HPA) axis and neuronal changes after adrenalectomy in type 2 diabetes. J Chem Neuroanat. 2010; 40:130–139.

34. Yi SS, Hwang IK, Kim DW, Shin JH, Nam SM, Choi JH, Lee CH, Won MH, Seong JK, Yoon YS. The chronological characteristics of SOD1 activity and inflammatory response in the hippocampi of STZ-induced type 1 diabetic rats. Neurochem Res. 2011; 36:117–128.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download