Abstract
The height, width, and cross-sectional area of the vertebral canal and spinal cord along with the area ratio of spinal cord to vertebral canal in the cervical vertebra were evaluated in images obtained using computed tomography (CT). Measurements were taken at the cranial, middle, and caudal point of each cervical vertebra in eight clinically normal small breed dogs (two shih tzu, two miniature schnauzers, and four mixed breed), 10 beagles, and four German shepherds. CT myelography facilitated the delineation of the epidural space, subarachnoid space, and spinal cord except at the caudal portion of the 7th cervical vertebra. The spinal cord had a tendency to have a clear ventral border in the middle portion of the vertebral canal and lateral borders near both end plates. The height, width, and area of the vertebral canal and spinal cord in the cervical vertebra were increased as the size of dog increased. However, the ratio of the spinal cord area to vertebral canal area in the small dogs was higher than that of the larger dogs. Results of the present study could provide basic and quantitative information for CT evaluation of pathologic lesions in the cervical vertebra and spinal cord.
The vertebra consists of a bony portion including the vertebral body, laminae, pedicles, and processes along with soft tissues including the spinal cord, nerves, vessels, and ligaments [8]. Most vertebral disorders are caused by damages to the spinal cord, which make the spinal cord compressed or swollen [24]. Wobbler's syndrome, intervertebral disk disease, hydromyelia, syringomyelia, intramedullary neoplasia, myelomalacia, and spinal cord atrophy are examples of spinal disorders [10,13,23,26].
Radiography is the primary diagnostic modality used to diagnose vertebral diseases in practice [18,21]. While basic radiography and standard myelography provide important information regarding the subarachnoid space, spinal cord, and vertebral canal, the complex shape of the vertebra as well as overlapping bony and soft tissue structures make radiographic evaluation of the vertebral column difficult [16]. Diagnostic imaging techniques used to visualize transverse planes such as computed tomography (CT) and magnetic resonance imaging (MRI) can reduce confusion encountered when evaluating vertebrae and the spinal cord by eliminating the superimposition of normal structures and pathological lesions. CT images can be displayed in different gray scale formats using various window setting according to the region of interest, and provide reformatted images that can enhance the visualization of specific structures without additional movement of the patient or further exposure to radiation [4,7,25].
Quantitative evaluation of spinal cord size along with the relationship between the spinal cord and vertebral canal can provide important data for distinguishing different diseases and predicting a patient's prognosis. Previous studies have evaluated the ratios of the diameter or area between spinal cord and vertebral canal in specific vertebral regions in dogs according to body size [5-6,9,12]. Data from these studies supported the conclusion that evaluation of the spinal cord, subarachnoid space, epidural space, and vertebral canal should performed based on the variety of breed-specific characteristics because there are significant differences in the component proportion ratio of subarachnoid space, spinal cord, and vertebral canal.
The cervical vertebra has been examined less frequently than the lumbar region in small dogs. However, accurate delineation of the spinal cord, subarachnoid space, and spinal canal in the cervical region is important for suspected cases of cervical intervertebral disk disease, spinal tumors, or syringohydromyelia because characteristic changes of the diameter or area ratio between spinal cord and vertebral canal can provide useful information for differentiating these cervical diseases. The ratio of the diameter of spinal cord to that of vertebral canal in the cervical vertebra has been previously evaluated using radiographic myelography [6]. In the present study, we measured the normal range of spinal cord dimensions including height, width, and area, and the ratio of spinal cord to vertebral canal area in canines using CT myelography. We hypothesized that the height, width, and ratio of spinal cord to vertebral canal of the cervical vertebra may vary according to body size. This anatomic distinction between the spinal cord and vertebral canal can be more clearly delineated using CT myelography to allow quantitative and objective evaluation of the cervical vertebra. The aim of this study was to identify the normal height, weight, and area of the spinal cord as well as the mean area ratio of spinal cord to vertebral canal area in the cervical vertebra of dogs.
Twenty-two adult dogs ranging from 2 to 6 years old were enrolled in this study. All animals were clinically healthy based on physical and neurological examinations, complete blood cell counts, serum biochemistry, and urine and cerebrospinal fluid analyses. The dogs were divided into three groups according to body weight. Group 1 included eight small dogs up to 10 kg (two shih tzu, two miniature schnauzers, and four mixed breed). Group 2 consisted of 10 beagles weighing 10~20 kg, and group 3 included four German shepherds over 20 kg. All dogs were fed commercial food and tap water ad libitum, and fasted 24 h prior to CT myelography. The canines were cared for according to The Guide for the Care and Use of Laboratory Animals of Seoul National University (Korea).
Myelography was performed under aseptic conditions. General anesthesia was induced with 0.5 mg/kg diazepam (Merode; Donghwa, Korea) and 6 mg/kg propofol (Pofol; Dongkook, Korea) delivered intravenously, and maintained with isoflurane (Isoflurane; Rhodia Oranique Fine, UK). With the dogs in a lateral recumbent position, cervical myelography was performed by injecting contrast medium, 0.3 mL/kg of iohexol (300 mgI/mL Omnipaque; Amersham Health, Ireland), into the cerebellomedullary cistern through a 22-gauge spinal needle (B-Braun Melsungen, Korea).
The dogs were positioned in a dorsal recumbent position with the forelimbs pulled cranially. Curvature of the vertebral column was minimized using sponge pads. CT images of the cervical vertebra were acquired using a CT-e unit (GE Medical Systems, Japan). Helical scanning was performed at 120 kVp and 60 mAs with 2 mm of thickness for groups 1 and 2, and 3 mm for group 3. All scans were performed with a 1.5-mm pitch factor.
CT images were evaluated at the bone window (window width = 2,000 HU, window level = 350 HU) for the vertebral canal and soft tissue window (window width = 400 HU, window level = 30 HU) for the spinal cord. The images were independently analyzed at the workstation by three radiologists (EJ Seo, JH Choi, and JH Yoon). The images were presented arbitrarily to the reviewers who were unaware of any image-associated information other than the window setting.
CT images of the cervical vertebra were evaluated for anatomic and quantitative analyses. The first and second cervical vertebrae (C1 and C2) were excluded because of the morphology was quite distinct from that of the other cervical vertebrae. For anatomic analysis, the spinal cord, subarachnoid space, epidural space, and vertebral canal were assessed from the third to seventh cervical vertebras while manipulating the window level and width. For quantitative analysis, the sagittal image was reformatted from the transverse plane and three points were selected for measurements in the cranial, middle, and caudal portions of the individual vertebra (Fig. 1). The cranial point was immediately caudal to the cranial end plate and the caudal point was immediately cranial to the caudal end plate. The middle point was located in the narrowest portion of the individual vertebral body. The height, width, and area of the spinal cord and vertebral canal were measured at each point, and the ratio of the spinal cord to vertebral canal area was calculated (Fig. 2). All measurements were made directly from the CT images using the standard internal measurement device of the CT scanner. To assess the reproducibility of the values, the spinal cord and vertebral canal areas were independently measured twice by the same three radiologists.
Statistical analysis was performed using the SPSS statistical computer program (SPSS ver. 12.0; SPSS, USA). According to the characteristics of the sample, a one-way analysis of variance (ANOVA) and Scheffe post hoc test were performed. Intraclass and interclass correlation coefficients were calculated according to the following formula:
Correlation coefficient = (variance between dogs-variance within dog)/(variance between dogs+variance within dog)
Variance of correlation coefficients was determined by using the ANOVA table. P-values less than 0.05 were considered statistically significant.
In the transverse images, the subarachnoid space was hyperattenuated with contrast medium. This change made the spinal cord and epidural space identifiable. Most of the portion displayed on the CT images showed excellent delineation except for the caudal part of the seventh cervical vertebra. The ventral and lateral margins of the spinal cord were clearly visualized from the cranial to caudal end plates. In particular, the spinal cord had a clear ventral border in the middle portion of the vertebral canal and lateral borders near both end plates. However, the dorsal area between the spinal cord and vertebral canal was too small to distinguish the distinct dorsal margin of the spinal cord. There was more space in the middle point of each vertebra and both lateral sides near the end plates according to the points measured in each vertebra. However, this was not statistically significant (p > 0.05).
The measured height, width, and area of the spinal cord and vertebral canal are presented as mean values for each point in Table 1 and Table 2. All values for the third through the seventh cervical vertebras, which were height, width, and area of the spinal cord and vertebral canal areas, tended to be greater as the size of dog increased (p < 0.05). The mean ratios of the spinal cord area to vertebral canal area measured at each point are shown in Table 3. Small dogs had a higher cord to canal ratio in the cervical vertebra than larger animals (p < 0.05). The mean ratios of the spinal cord area to vertebral canal area in each cervical vertebra regardless of measuring points are shown in Table 4. Values of the spinal cord and vertebral canal areas taken with repeated measurements were not significantly different (p > 0.05). Likewise, intraclass correlation coefficients were not significantly different (p > 0.05).
Various spinal and vertebral diseases including intervertebral disk disease, syringohydromyelia, Wobber's syndrome, neoplasia, and spinal cord atrophy can occur in cervical regions. The spinal cord may be characteristically altered according to the disease. For example, spinal cord tumors are associated with increased diameter and area of the spinal cord that can lead an increased ratio of spinal cord to vertebral canal area [4]. The compressed spinal area of the vertebral canal in cases of intervertebral disk disease cases may have a clinical correlation with symptoms or prognosis [15]. In cases of syringomyelia, the cervical vertebral canal diameter is significantly greater than that in the normal control with a widened spinal cord [14]. Therefore, delineation between the bony structure and spinal cord in vertebrae is important to establish reliable differential diagnostic techniques.
CT and MRI can define the bony and soft tissue structures more clearly compared to radiographic myelography. MRI can provide a higher contrast within soft tissues compared to CT [2]. However, MRI is less available than CT in veterinary medicine. In human medicine, the normal range of cross-sectional diameter and spinal cord area has been established, and correlation between alterations of the spinal cord area and clinical signs has been assessed with CT myelography in the cervical vertebra [20,22]. In dogs, the normal range of the cervical spinal cord to vertebral canal area ratio was proposed using radiographic myelography in small and large animals [6]. The vertebral canal and body ratio as well as morphologic features of the cervical vertebra have been investigated in studies of Doberman pinschers focused on cervical spondylomeylopathy [1,3]. However, no investigation has been performed using trans-sectional imaging modalities such as CT or MRI to determine the spinal cord to vertebral canal area ratio in the cervical region of normal dogs.
CT myelography can help delineate the spinal cord, particularly in small dogs. This can improve the visualization of anatomic details in cases of spinal diseases [11]. Margins of the spinal cord, subarachnoid space, and epidural space were clearly observed using CT myelography in the present study. The height, width, and areas of the spinal cord and vertebral canal were evaluated at three points located in the cranial, middle, and caudal regions of the cervical vertebra body. The space in the ventral region at the middle point of each vertebra and in both lateral sides near the end plates tended to be greater even though these differences were not statistically significant.
Variations of the vertebral and spinal cord structures according to body size have been reported in the literature [6,12,17]. In small dogs, the subarachnoid space is relatively narrow and conforms closely to bones forming the vertebral canal in the thoracolumbar region. In contrast, large breeds tend to have relatively thinner spinal cords and a uniform, parallel-sided subarachnoid space [12]. In the present study, the height, width, and area measured in the three groups of dogs tended to increase as the size of the dog increased. This result concurs with the finding that vertebral canal height in German shepherds is greater throughout the length of the vertebral column compared to that of dachshunds in the thoracolumbar region [17] even though no data was obtained from the cervical region.
In the present study, the ratio of the spinal cord area to vertebral canal area was greater in small dogs compared to large dogs in this study. This result coincides with findings from a previous study in which radiographic myelography in cervical vertebra was performed to evaluate similar variables except for height [6]. The authors proposed the possibility that the anatomic difference between small and large dogs is due to small extradural compression in small dogs with larger spinal cord to vertebral canal area ratios. This characteristic is clinically significance and can cause neck pain because small dogs have a relatively narrower subarachnoid space in the cervical region [6]. This feature was reported in the thoracolumbar region in a previous study that compared the spinal cord to the vertebral canal ratio between German shepherds and dachshunds, and demonstrated that the ratio in German shepherds was consistently smaller than that in dachshund [17].
We used the relative measurement method based on ratios of spinal cord to vertebral canal area in the present study. The relative measurement technique has improved the accuracy of diagnosing spinal diseases including cervical spinal stenosis in humans [19], and is independent from magnification factors. In our investigation, cross-sectional areas of the spinal cord and vertebral canal were used instead of height based on a previous CT study of intervertebral disk disease in dogs [15] in which clinical grade correlated with the area ratio of herniated disc material to the spinal cord, but not with the height ratio.
A limitation of our study was that we established three separate experimental groups: small, middle, and large animals. The number of large dogs we evaluated was too small to analyze statistically, and the Kurskal-Wallis test was used as a non-parametric test. There was a considerable weight distribution gap among each group. Future studies will be required to investigate comprehensive trends excluding that kind of a gap observed in the present study. Moreover, correlations between the degree of cervical spinal cord compression based on the ratio of spinal cord area to vertebral canal area as well as the clinical significance need to be further assessed.
Despite the above limitation, the present study provides fundamental morphometric information and insight for evaluating the cervical vertebra of normal dogs. The cranial cervical region as well as the caudal region including the seventh vertebra were assessed in the present study. Thus, our investigation provided useful anatomical information for dogs suspected to have cord atrophy or cord involvement with marked spondylosis deformans in the caudal cervical area. Furthermore, the normal area ratio observed in this study can be used for quantitative and objective evaluation of cervical spinal cord size and early identification of alterations in the cervical spinal cord diameter.
Figures and Tables
Fig. 1
Reformatted sagittal image of the fourth cervical vertebra derived from the initial transverse image. Arrows indicate the cranial (Cr), middle (M), and caudal (Cd) points that were used to measure the height, width, and area of the spinal cord and vertebral canal.
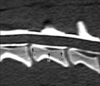
Fig. 2
Height, width, and area of the vertebral canal and spinal cord in the middle portion of the fourth cervical vertebra were measured on the images in which the vertebra window (A) and vertebra window (B) were manipulated.
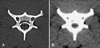
Table 1
Mean vertebral canal height and width, and cervical vertebral area in normal dogs

Data are expressed as the mean ± standard deviation (SD). Units of measurement are mm for height and width, and mm2 for area. C, cervical vertebra; Cranial point, immediately caudal to cranial end-plate; Middle point, the narrowest portion of the individual vertebral body; Caudal point, immediately cranial to the caudal end plate.
References
1. da Costa RC, Parent JM, Partlow G, Dobson H, Holmberg DL, LaMarre J. Morphologic and morphometric magnetic resonance imaging features of Doberman Pinschers with and without clinical signs of cervical spondylomyelopathy. Am J Vet Res. 2006; 67:1601–1612.

2. de Decker S, Gielen IMVL, Duchateau L, Polis I, van Bree HJJ, van Ham LML. Agreement and repeatability of linear vertebral body and canal measurements using computed tomography (CT) and low field magnetic resonance imaging (MRI). Vet Surg. 2010; 39:28–34.

3. de Decker S, Gielen IMVL, Duchateau L, Saunders JHH, van Bree HJJ, Polis I, van Ham LML. Magnetic resonance imaging vertebral canal and body ratios in Doberman Pinschers with and without disk-associated cervical spondylomyelopathy and clinically normal English Foxhounds. Am J Vet Res. 2011; 72:1496–1504.

4. Drost WT, Love NE, Berry CR. Comparison of radiography, myelography and computed tomography for the evaluation of canine vertebral and spinal cord tumors in sixteen dogs. Vet Radiol Ultrasound. 1996; 37:28–33.

5. Feeney DA, Evers P, Fletcher TF, Hardy RM, Wallace LJ. Computed tomography of the normal canine lumbosarcral spine: a morphologic perspective. Vet Radiol Ultrasound. 1996; 37:399–411.

6. Fourie SL, Kirberger RM. Relationship of cervical spinal cord diameter to vertebral dimensions: a radiographic study of normal dogs. Vet Radiol Ultrasound. 1999; 40:137–143.

8. Haughton VM, Syvertsen A, Williams AL. Soft-tissue anatomy within the spinal canal as seen on computed tomography. Radiology. 1980; 134:649–655.

9. Hecht S, Huerta MM, Reed RB. Magnetic resonance imaging (MRI) spinal cord and canal measurements in normal dogs. Anat Histol Embryol. 2014; 43:36–41.

10. Itoh T, Nishimura R, Matsunaga S, Kadosawa T, Mochizuki M, Sasaki N. Syringomyelia and hydrocephalus in a dog. J Am Vet Med Assoc. 1996; 209:934–936.
11. Ketonen L, Gyldensted C. Lumbar disc disease evaluated by myelography and postmyelography spinal computed tomography. Neuroradiology. 1986; 28:144–149.

12. Lamb CR. Common difficulties with myelographic diagnosis of acute intervertebral disc prolapse in the dog. J Small Anim Pract. 1994; 35:549–558.

13. Lee BC, Kazam E, Newman AD. Computed tomography of the spine and spinal cord. Radiology. 1978; 128:95–102.

14. Lee J, Williams B. Cervical vertebrae measurements in syringomyelia. Clin Radiol. 1977; 28:395–400.

15. Lim C, Kweon OK, Choi MC, Choi J, Yoon J. Computed tomographic characteristics of acute thoracolumbar intervertebral disc disease in dogs. J Vet Sci. 2010; 11:73–79.

16. Morgan JP, Ackerman N, Bailey CS, Pool RR. Vertebral tumors in the dog: a clinical radiologic, and pathologic study of 61 primary and secondary lesions. Veterinary Radiology. 1980; 21:197–212.

17. Morgan JP, Atilola M, Bailey CS. Vertebral canal and spinal cord mensuration: a comparative study of its effect on lumbosacral myelography in the Dachshund and German shepherd dog. J Am Vet Med Assoc. 1987; 191:951–957.
18. Morgan JP, Miyabayashi T, Choy S. Cervical spine motion: radiographic study. Am J Vet Res. 1986; 47:2165–2169.
19. Pavlov H, Torg JS, Robie B, Jahre C. Cervical spinal stenosis: determination with vertebral body ratio method. Radiology. 1987; 164:771–775.

20. Penning L, Wilmink JT, van Woerden HH, Knol E. CT myelographic findings in degenerative disorders of the cervical spine: clinical significance. AJR Am J Roentgenol. 1986; 146:793–801.

21. Sande RD. Radiography, myelography, computed tomography and magnetic imaging of the spine. Vet Clin North Am Small Anim Pract. 1992; 22:811–831.

22. Stanley JH, Schabel SI, Frey GD, Hungerford GD. Quantitative analysis of the cervical spinal canal by computed tomography. Neuroradiology. 1986; 28:139–143.

23. Tadmor R, Cacayorin ED, Kieffer SA. Advantages supplementary CT in myelography of intraspinal masses. AJNR Am J Neuroradiol. 1983; 4:618–621.
24. Tidwell AS, Solano M, Schelling SH. Pediatric neuroimaging. Semin Vet Med Surg (Small Anim). 1994; 9:68–85.
25. Wortman JA. Principles of X-ray computed tomography and magnetic resonance imaging. Semin Vet Med Surg (Small Anim). 1986; 1:176–184.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download