Abstract
Figures and Tables
 | Fig. 1Scanned image of antibody array slides. Proteins were extracted from livers of mice fed a normal diet (control), high fat diet (HF), and high fat diet with resveratrol (HFR). Proteins extracted from each group (n = 12) were combined, and phosphorylation levels were analyzed using an antibody array. Each slide consists of an array of antibodies with six replicates per antibody, and multiple positive and negative controls to maximize data quality and reproducibility. Signals from three different array chips were normalized based on the GAPDH signal after background subtraction. |
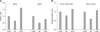 | Fig. 2Restoration effects of resveratrol on protein phosphorylation in the insulin signaling pathway of obese mice. (A) The phosphorylation levels of IRS-1 (Ser636, Ser639). (B) The levels of PI3-kinase p85-subunit α/γ (Tyr467/Tyr199) and PDK1 (Ser241) among the control, HF, and HFR groups were compared. Proteins extracted from each group (n = 12) were combined, and phosphorylation levels were analyzed using the antibody array. |
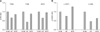 | Fig. 3Restoration effects of resveratrol on the phosphorylation of Akt and GSK3 of obese mice. (A) The phosphorylation levels of Akt (Thr308, Tyr326, Ser473). (B) The levels of GSK-3α (S21) and GSK-3 (Ser9) among the control, HF, and HFR groups were compared. Proteins extracted from each group (n = 12) were combined, and phosphorylation levels were analyzed using the antibody array. |
 | Fig. 4Effects of resveratrol on protein phosphorylation in the insulin signaling pathway and the mTOR pathway of obese mice. |
Table 2
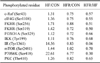
c-Raf: proto-oncogene serine/threonine-protein kinase, eIF4G: eukaryotic translation initiation factor 4G, FKHR: forkhead in human rhabdomyosarcoma, FOXO1A: forkhead box protein 1A, IKK: I-κB kinase, IR: insulin receptor, mTOR: mammalian target of rafamycin, P70S6K: serine/threonine kinase regulated by PI3K/mTOR pathway, PKC: protein kinase C.
Table 3

AMPK1: 5 adenosine monophosphate-activated protein kinase, GSK: glycogen synthase kinase, HSL: hormone-sensitive lipase, IRS: insulin receptor substrate, MEK: MAPkinase kinase, PDK: PIP3-dependent kinase, PI3K: phosphoinositide 3-kinase, PP2A: protein phosphatase 2, PTEN: phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase, SHP: Src homology region 2 domain-containing phosphatase.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download