Abstract
The level of P4 at the time of embryo transfer (ET) is important. P4 concentrations and numbers of corpora lutea for 126 recipients were evaluated. Nuclear transfer embryos were transferred into 126 surrogates. 11 maintained their pregnancy until full-term delivery, 17 miscarried, and implantation failed in 98 animals. P4 levels in the full-term group were significantly different from those of the pigs that aborted or in which implantation failed (p < 0.05). However, the numbers of corpora lutea were not significantly different. These findings indicate that the concentration of progesterone can be an important factor for successful ET in pigs.
The normal estrus cycle in pigs lasts 18~24 days including 5~7 days of a follicular phase and 13~15 days of a luteal phase [20]. The reproductive physiology of sows is affected by various factors including the environment [3,7], hormones [10,15,16], diet [4,9], and genetics [5,13]. These factors are important not only for increasing litter sizes on commercial farms but also improving embryo production by somatic cell nuclear transfer (SCNT) in the laboratory.
Progesterone (P4) is important for early implantation and pregnancy maintenance [6]. This factor is secreted from ovaries by the corpus luteum (CL) [2] and uteri by the myometrium [19]. After ovulation, P4 levels gradually increase as the CLs develop. Peak concentrations (approximately 20 ng/mL) are reached 8~9 days after ovulation [14,17]. The numbers of CLs in a single ovary, which are easily determined during embryo transfer (ET), are also important. In gilts, the rates of blastocyst formation and P4 concentrations in follicular fluid seem to correlate with the CL numbers in each ovary. Thus, the numbers of CLs in the ovary could serve as an indicator for estimating the developmental competence of porcine oocytes following embryo transfer [20].
One of the causes of low SCNT embryo implantation and term delivery rates is the timing of ET. For this retrospective analysis, we divided recipient sows into three groups. One group was comprised of animals that achieved full pregnancy and term delivery, another group included pigs that experienced aborted pregnancies, and the third group consisted of recipients in which implantation failed. We estimated P4 concentrations and the numbers of CLs at the time of ET in all recipients. We hypothesized that this information could help to determine the appropriate time for ET and thereby increase the delivery rate of SCNT-derived embryos.
All chemicals were obtained from Sigma-Aldrich Corp. (USA) unless otherwise stated. Protocols involving the use of animals in this study were approved (12-2009-008-5) by the Institutional Animal Care and Use Committee of Seoul National University (Korea). The study was also conducted in accordance with the Guide for the Care and Use of Laboratory Animals of Seoul National University (Korea).
SCNT was performed according to a protocol previously established in our laboratory [12]. Pig ovaries were collected from a local abattoir (Gimpo, Gyeonggi-do, Korea) and transported to the laboratory within 3 h in physiological saline. Follicular fluid was aspirated from follicles 3 to 6 mm in diameter using an 18-gauge needle attached to a 10-mL syringe (Hwajin, Korea), transferred to 50-mL conical tubes (Catalog No. 50050; SPL Life Sciences, Korea), and incubated at 39℃. After 15 min of sedimentation, the supernatant was discarded. The sediments were transferred to a 100-mm Petri dish (Catalog No.10090; SPL Life Sciences) and washed with physiological saline containing 1% (v/v) penicillin/streptomycin.
Cumulus-oocyte complexes (COCs) with intact, unexpanded cumulus cell layers and homogeneous cytoplasm were selected for further culturing. The COCs were cultured with in vitro maturation (IVM) medium at 39℃ under 5% CO2 in air for 22 h with 0.5 µg/mL of Follicle stimulating hormone (FSH) and 0.5 µg/mL of Luteinizing hormone (LH). Next, the COCs were washed with Medium 199, transferred to hormone-free IVM medium, and cultured at 39℃ under 5% CO2 and 5% O2 in air for another 22 h for final oocyte maturation. Cumulus cells were removed from the oocytes by repeated pipetting in tyrode lactose (TL) medium supplemented with 10 mM HEPES and 0.3% (w/v) polyvinylpyrrolidone (PVP) (HEPES-TL-PVP) medium [8] with 1 mg/mL hyaluronidase. The IVM oocytes were then enucleated using an aspiration pipette (Catalog No. MSC-20-0; Origio, Denmark), microinjected with a fetal fibroblast as a donor cell, and then fused by electrical stimulation with electro cell fusion machine (Catalog No. LF101; Sonidel Limited, USA). Finally, the artificially constructed embryos were activated with a single 1.5 kV/cm DC electric pulse for 60 µsec using a BTX Electro-Cell Manipulator 2001 (BTX, USA). Only the cleaved embryos were transferred into surrogate mothers within 2 days.
The surrogate mother was restrained and anesthesia was induced by injection of ketamine (10 mg/kg; Yuhan, Korea) and xylazine (1 mg/kg; Bayer, USA) into an ear vein as previously described [18]. After intravenous injection, the unconscious pig was placed on a surgery table in a ventro-dorsal posture. General anesthesia was maintained with isoflurane (Hana Pharm, Korea) under the supervision of a veterinarian.
While under general anesthesia, blood samples were collected from the jugular veins of 126 surrogate pigs using 18-gauge needles connected to disposable syringes. The samples were put into serum-separating tubes (BD Biosciences, USA), centrifuged 5,000 × g 10 min at 25℃ to separate serum from blood after clotting, and then delivered to the laboratory at 0℃ in an ice box. The samples were then transported to an analysis center (Neodin Medical Institute, Korea) to measure the P4 concentration.
Reconstructed embryos were loaded into a Tomcat catheter (Catalog No. 8890703021; Covidien, USA) with HEPES-TL-PVP medium equilibrated in 5% CO2 with air. Only 2- or 4-cell stage embryos were transferred. The embryos were placed into the uterine tubes of each surrogate animal through a small puncture made with a suture needle (Catalog No. 6307-71; Covidien).
Data were analyzed with a one-way analysis of variance (ANOVA) followed by Dunn's test. All analyses were performed with GraphPad Prism version 5.01 (GraphPad Software, USA) to identify differences among the experimental groups. The p values less than 0.05 were considered statistically significant.
In this study, a total of 126 recipients were examined. Eleven (8.7%) experienced full-term delivery, 17 (13.5%) recipients aborted, and implantation failed in 98 (77.8%) recipients (Table 1 and Supplementary Table 1). P4 levels were 1.99 ± 0.17 ng/mL for the full term-delivery group. This was significantly different from the concentrations observed for the two other groups (4.04 ± 0.17 ng/mL for the aborted pregnancy group and 4.73 ± 0.04 ng/mL for the implantation failure group; Fig. 1). The numbers of CLs ware 7.14 ± 0.15 for the full-term delivery group, 5.50 ± 0.13 for the aborted pregnancy group, and 7.03 ± 0.02 for the implantation failure group (Fig. 2). No significant differences were found among the three groups.
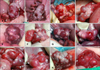
Photographs were taken of all ovaries to further examine the relationship between morphological differences and reproduction rates. No distinct differences were found among the three groups. Non-ovulated follicles were detected in the full-term delivery group, similar to the other two groups (Fig. 3D). Ovulated follicles were commonly observed in the aborted pregnancy and implantation failure groups. Non-ovulated follicles were also found in the aborted pregnancy (Figs. 3E, F, and G) and implantation failure groups (Figs. 3I, J, and L).
Blood sample collection and the estimation of P4 concentrations 1~2 days before embryo transfer are important tools. However, blood sampling from the jugular vein of full-grown pigs is stressful even after the animals have undergone several trials. These stressors activate the hypothalamus-pituitary-adrenal axis and increase the release of peptides from the hypothalamus, principally corticotropin-releasing hormone that induces adrenocorticotropic hormone (ACTH) release. ACTH stimulates the adrenal cortex to secrete corticosteroids. Consequently, prolonged or chronic stress commonly inhibits reproduction while transient or acute stress might be causative factors that occasionally disrupt reproduction [8]. To help reduce stress for ET surrogates, P4 concentrations were estimated just before ET was performed under general anesthesia. Thus, these P4 concentrations could not be used as a reference for selecting appropriate recipients. However, the cumulative data and experiences encouraged us to select suitable surrogates without blood sampling by correlating P4 concentrations with external estrous signs such as redness of the vulva or standing estrus.
Robertson and King [14] described one gilt with substantially greater numbers of CLs that had extremely high P4 concentrations during early pregnancy which decreased sharply to normal ranges during late pregnancy. A similar finding was reported in goats by Jarrell and Dziuk [1]. In the present study, no significant relationship was found between the numbers of CLs and pregnancy status. To reduce the duration of ET surgeries while lowering stress and preventing injury to the surrogates, the numbers of CLs were determined in only one ovary. According to Joh et al. [11], the numbers of CLs are approximately the same in both ovaries.
In summary, we demonstrated that P4 concentration at the time of ET is a critical factor can affect the success of reproduction using SCNT embryos. In contrast, the numbers of CLs were not contributing factors for ET success. Application of these findings for selecting surrogates may help improve pregnancy rates after the transfer of SCNT embryos.
Figures and Tables
Fig. 1
Progesterone (P4) concentrations of the three groups of surrogates (term-delivered, aborted, and implantation failure) at the time of embryo transfer (ET). The levels of P4 were 1.99 ± 0.17 ng/mL, 4.04 ± 0.17 ng/mL, and 4.73 ± 0.04 ng/mL for the three groups, respectively. The full-term delivery group had a significantly lower P4 concentration compared to that of the other two groups.

Fig. 2
Numbers of corpora lutea (CLs) in the three groups of surrogate pigs (term-delivered, aborted, and implantation failure). The numbers of CLs were 7.14 ± 0.15, 5.50 ± 0.13, and 7.03 ± 0.02 for the three groups, respectively. There were no significant differences among the groups.

Fig. 3
Ovaries of the recipients at the time of ET. (A~D) Ovaries from full-term delivered recipients. (E~H) Ovaries from aborted pregnancy animals. (I~L) Ovaries from recipients that experienced implantation failure. Non-ovulated follicles were detected even in recipients that successfully delivered offspring (D). While ovulated follicles were commonly detected in the aborted and implantation-failure groups, non-ovulated follicles were also found in the aborted (E, F, and G) and implantation-failure animals (I, J and L). White arrows indicate the non-ovulated follicles.
Acknowledgments
This study was supported by Ministry of Trade, Industry and Energy (No. 10033839), Korea Institute of Planining & Evaluation for Technology (No. 311011-05-3-SB010), and the TS Corporation, Korea. We also thank Mr. YoonSang Kweon for his excellent administrative support.
References
1. Aba MA, Sumar J, Kindahl H, Forsberg M, Edqvist LE. Plasma concentrations of 15-ketodihydro-PGF2α, progesterone, oestrone sulphate, oestradiol-17β and cortisol during late gestation, parturition and the early post partum period in llamas and alpacas. Anim Reprod Sci. 1998; 50:111–121.

2. Adair V, Stromer MH, Anderson LL. Progesterone secretion and mitochondrial size of aging porcine corpora lutea. Anat Rec. 1989; 223:252–256.

3. Bertoldo MJ, Holyoake PK, Evans G, Grupen CG. Seasonal variation in the ovarian function of sows. Reprod Fertil Dev. 2012; 24:822–834.

4. Chen TY, Stott P, Athorn RZ, Bouwman EG, Langendijk P. Undernutrition during early follicle development has irreversible effects on ovulation rate and embryos. Reprod Fertil Dev. 2012; 24:886–892.

5. Coster A, Madsen O, Heuven HCM, Dibbits B, Groenen MAM, van Arendonk JAM, Bovenhuis H. The imprinted gene DIO3 is a candidate gene for litter size in pigs. PLoS One. 2012; 7:e31825.

6. Dai B, Cao Y, Liu W, Li S, Yang Y, Chen D, Duan E. Dual roles of progesterone in embryo implantation in mouse. Endocrine. 2003; 21:123–132.

7. De Rensis F, Saleri R, Tummaruk P, Techakumphu M, Kirkwood RN. Prostaglandin F2α and control of reproduction in female swine: a review. Theriogenology. 2012; 77:1–11.

8. Esaki R, Ueda H, Kurome M, Hirakawa K, Tomii R, Yoshioka H, Ushijima H, Kuwayama M, Nagashima H. Cryopreservation of porcine embryos derived from in vitro-matured oocytes. Biol Reprod. 2004; 71:432–437.

9. Gao K, Jiang Z, Lin Y, Zheng C, Zhou G, Chen F, Yang L, Wu G. Dietary L-arginine supplementation enhances placental growth and reproductive performance in sows. Amino Acids. 2012; 42:2207–2214.

10. Gonzalez-Bulnes A, Torres-Rovira L, Ovilo C, Astiz S, Gomez-Izquierdo E, Gonzalez-Añover P, Pallares P, Perez-Solana ML, Sanchez-Sanchez R. Reproductive, endocrine and metabolic feto-maternal features and placental gene expression in a swine breed with obesity/leptin resistance. Gen Comp Endocrinol. 2012; 176:94–101.

11. Joh CH, Kim YJ, Shin ST, Son CH, Lee BC, Lee ES, Rho GJ, Kim IH, Kang TY, Kang HG, Park JI, Cho JK, Yoo IJ, Oh KS, Kim SJ, Lee SL, Jang G. Veterinary obstetrics and theriogenology. Seoul: HongYoungSa;2010. p. 146.
12. Koo OJ, Park HJ, Kwon DK, Kang JT, Jang G, Lee BC. Effect of recipient breed on delivery rate of cloned miniature pig. Zygote. 2009; 17:203–207.

13. Rátky J, Brüssow KP, Egerszegi I, Torner H, Schneider F, Solti L, Manabe N. Comparison of follicular and oocyte development and reproductive hormone secretion during the ovulatory period in Hungarian native breed, Mangalica, and Landrace gilts. J Reprod Dev. 2005; 51:427–432.

14. Robertson HA, King GJ. Plasma concentrations of progesterone, oestrone, oestradiol-17β and of oestrone sulphate in the pig at implantation, during pregnancy and at parturition. J Reprod Fertil. 1974; 40:133–141.

15. Ropka-Molik K, Oczkowicz M, Mucha A, Piórkowska K, PiestrzyXMLLink_XYZska-Kajtoch A. Variability of mRNA abundance of leukemia inhibitory factor gene (LIF) in porcine ovary, oviduct and uterus tissues. Mol Biol Rep. 2012; 39:7965–7972.

16. Siawrys G, Smolinska N. Direct in vitro effect of LH and steroids on leptin gene expression and leptin secretion by porcine luteal cells during the mid-luteal phase of the estrous cycle. Reprod Biol. 2012; 12:317–323.

17. Soede NM, Langendijk P, Kemp B. Reproductive cycles in pigs. Anim Reprod Sci. 2011; 124:251–258.

18. Trim CM, Gilroy BA. Cardiopulmonary effects of a xylazine and ketamine combination in pigs. Res Vet Sci. 1985; 38:30–34.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download