Introduction
Coronary flow velocity and coronary flow reserve measurements provide useful clinical and physiological information in humans [17]. Until recently, coronary artery disease was conventionally evaluated by assessing regional and global left ventricular function with the patient at rest and under stress [34]. Imaging techniques that enable direct visualization of the coronary arteries include Doppler guidewire [1], computed tomographic angiography [22,24,25], transesophageal [33] or intracardiac echocardiography [30], and magnetic resonance angiography [3,20]. These techniques are invasive and expensive [34], and their use in veterinary medicine is therefore limited.
The measurement of coronary flow velocity using transthoracic Doppler echocardiography was first described in human medicine in 1987 [34]. Since then, visualization of the coronary arteries and blood flow assessment using transthoracic echocardiography has improved owing to technological advances in ultrasonography with the aid of digital imaging using a high-frequency transducer [34]. Transthoracic echocardiography is a noninvasive, inexpensive, and reproducible technique for monitoring coronary arterial flow [17]. However, transthoracic echocardiography for evaluating coronary flow velocities has not been utilized in veterinary medicine to the best of our knowledge. The purpose of this study was to evaluate the ability of echocardiographic techniques to measure peak diastolic velocity in the coronary arteries of clinically healthy beagles. In addition, coronary artery flow in normal dogs was compared to that observed in a canine model of myocardial infarction (MI).
Go to : 
Materials and Methods
The Institutional Animal Care and Use Committee of Konkuk University (Korea) approved the study protocol (IACUC No. KU11059). In total, 17 beagles were included in the investigation: 11 were in the control group (seven males and four females) and were six included in the MI group (four males and two females). All dogs were purchased from Beijing Marshall Biotechnology (China). The median weight of the dogs was 9.43 kg (range: 8.5~10.5 kg) and the median age was 22.8 months (range: 15~28 months). Following clinical and laboratory examinations, all the dogs were found to be in good health. Results from the physical examination, hematology, serology, electrocardiography, radiography, and echocardiography were all normal. The dogs were also negative for heartworm infections according to the Dirofilaria immitis antigen test and microscopy evaluation of blood samples.
Six canines in the MI group had previously undergone surgical ligation of the left anterior descending (LAD) artery below the first diagonal branch. Medetomidine (0.02 mg/kg, intravenously, Domitor; Oricon Corporation Animal Health, Finland) was used for sedation 20 min before surgery and propofol (5 mg/kg, intravenously, Anepol; Hana Pharma, Korea) was administered to induce anesthesia. The dogs were intubated with an endotracheal tube and anesthesia was maintained with isoflorane (1.5~2% in 100% oxygen through the endotracheal tube, Foran; JW Pharmaceutical, Korea).
The dogs were placed in a right lateral recumbent position and left thoracotomy was performed at the level of the 5th intercostal space. After thoracotomy, the pericardial sac was incised and the exposed paraconal branch of the left coronary artery was permanently ligated using non-absorbable suture material (Prolene; Johnson & Johnson Medical, USA) below the first diagonal branch to induce MI. Lidocaine (Daehan Lidocaine HCl 2%; Daehan Pharma, Korea) was injected as a 2 mg/kg intravenous bolus before coronary artery ligation was performed. This compound was then administrated as an intravenous infusion at a constant rate of 0.05 mg/kg/min during the rest of the surgical procedure to prevent ventricular tachycardia. MI was confirmed by immediate ST segment elevation on electrocardiography and a color change of the myocardium to blue. After coronary artery ligation, the pericardium and thoracic wall was closed with absorbable suture material (Maxon; Covidien, Ireland). Tramadol (5 mg/kg, intravenously, Tridol amp.; Yuhan Corporation, Korea) and cephradine (30 mg/kg, intravenously, Cefradine; Sinil Pharm, Korea) were administrated for pain control and to prevent infection, respectively.
Echocardiography was performed using ultrasound (Prosound alpha7; Hitachi Aloka Medical, Japan) and a 3~8-MHz broad-frequency sector transducer a month after MI induction while the dogs were conscious. Ultrasonographic scanning was performed using 6.15 MHz color Doppler flow imaging. To obtain images of optimal quality, the velocity range and gain setting were adjusted accordingly. To make a complete assessment, both the left coronary artery (LCA) and right coronary artery (RCA) were examined in this study. To evaluate the LCA, all the dogs were examined while in a right lateral recumbent position and an acoustic window was created in the fourth or fifth intercostal spaces. If images of the aorta were obtained in the right parasternal long-axis left ventricular outflow view, then the area anterior to the left ventricular outflow tract and left interventricular sulcus were examined using 2D imaging in combination with color flow mapping. Images of the RCA were obtained with the transducer placed in the fourth and fifth intercostal spaces while the animals were in a left lateral recumbent position. When the transverse images of the aorta were identified in the left cranial parasternal short-axis view of the aorta, the right coronary cusp of the aorta was examined with 2D imaging and color flow mapping. Peak diastolic velocity and diastolic deceleration time of the RCAs and LCAs were measured by transthoracic pulsed-wave Doppler echocardiography with a sample volume of 1.5 mm and a frequency of 5.0 MHz.
All the data are expressed as the mean and standard deviation, and analyzed using statistical software (SPSS for Windows, ver. 19.0; SPSS, USA). An independent t-test with Bonferroni correction was used to compare the coronary artery diastolic peak velocities in each artery between the groups (control and MI dogs) and within each group (a p value < 0.01 was considered to be statistically significant). To evaluate the effect of observational variability on the measurement of diastolic peak velocity, two independent observers (Mr. Jaehwan Kim and Mr. Seungjun Lee) acquired images of coronary artery blood flow with pulsed wave Doppler echocardiography and recorded their results separately. Inter-observer agreement was assessed using two-way random intraclass coefficiency. Reproducibility was measured by conducting two transthoracic echocardiograph examinations 2 days apart for each dog. One-way intraclass coefficiency values were obtained to evaluate observed agreement. Inter- and intra-observer agreement was considered significant if the p value was less than 0.05.
Go to : 
Results
Before assessments of coronary arterial flow were made by transthoracic Doppler echocardiography, successful MI induction in all six MI dogs was confirmed by two-dimensional echocardiographic findings such as wall thinning, hyperechoic fibrosis, and hypokinesia of the infarcted segment of the left ventricular apex. However, other signs associated with MI, such as lethargy or exercise intolerance, were not observed in the MI group. After confirming MI induction by two-dimensional echocardiography, transthoracic echocardiographic evaluation of the coronary arteries was performed while the animals were conscious. To obtain images of the LCA, a clockwise-rotated beam angle (range: 28~47.7°) in the right parasternal long-axis left ventricular outflow view was used. For the RCA, a clockwise-rotated inclined beam angle correction (range: 28~44°) in the left cranial parasternal short-axis view of the aorta was employed.
Hypoechoic coronary arteries were distinct from other cardiac structures on the two dimensional echocardiography images. The LCA was an infundibular structure arising from the sinus of Valsalva on the right parasternal long-axis left ventricular outflow view. The RCA appeared as tubular structures with the thin hyperechoic wall arising from the right coronary cusp of the aorta on the left cranial parasternal short-axis view. Blood flow of the LCA moved away from the probe on the color Doppler echocardiography whereas blood flowed toward the probe in the RCA (Fig. 1). Systolic-(S) and diastolic-phase (D) waves for the RCA were found, but an S-wave was not observed in the LCA. Blood flow waves in the coronary arteries had rapid acceleration times and relatively long deceleration times (Fig. 2). The mean peak diastolic velocities of the LCAs and RCAs in the control group were 19.47 ± 2.67 and 20.84 ± 3.24 cm/sec, respectively (Table 1), and were not significantly different. Diastolic deceleration time of the LCA was slightly more rapid compared to that of the RCA (0.91 ± 0.14 sec vs. 1.13 ± 0.20 sec).
 | Fig. 1Images of the coronary arteries with two-dimensional echocardiography (left) and color Doppler mapping (right). (A) Image of the right coronary artery (RCA; arrows) obtained from the modified left cranial short-axis view. (B) Image of the left coronary artery (LCA; arrows) acquired from the modified right parasternal long-axis left ventricular outflow tract view. RV: right ventricle, RA: right atrium, Ao: aorta, LA: left atrium, LV: left ventricle. |
 | Fig. 2Spectral Doppler mode images of coronary blood flow in the myocardial infarction (MI) group and control group. (A) RCA blood flow in a control dog. (B) RCA blood flow in a beagle with MI. (C) LCA blood flow in a control canine. (D) LCA blood flow in an animal with MI. S: systolic wave, D: diastolic wave. |
In the MI group, peak diastolic velocities and diastolic deceleration times of the LCAs and RCAs were significantly different from those of the control group (Fig. 2 and Table 1). Peak diastolic velocity of the LCA was significantly lower compared to that of the RCA in the MI group. Moreover, peak diastolic velocity of the LCA in the MI group was significantly slower relative to that of the control group (14.82 ± 1.61 cm/sec vs. 19.47 ± 2.67 cm/sec) and peak diastolic velocity of RCA in the MI group was significantly faster than that in the control group (31.61 ± 2.34 cm/sec vs. 20.84 ± 3.24 cm/sec; Table 1, Figs. 2 and 3). Diastolic deceleration time was also altered in the MI group. Diastolic deceleration time of the LCA was slightly slower in MI group compared with that in the control group (1.02 ± 0.22 sec vs. 0.91 ± 0.14 sec). The MI group had a significantly faster diastolic deceleration time in the RCA than the control group (0.71 ± 0.06 sec vs. 1.13 ± 0.20 sec). Differences in diastolic deceleration time between the LCA and RCA were significant and diastolic deceleration time of the LCA was slower than that of the RCA (1.02 ± 0.22 sec vs. 0.71 ± 0.06 sec; Table 1, Figs. 2 and 4).
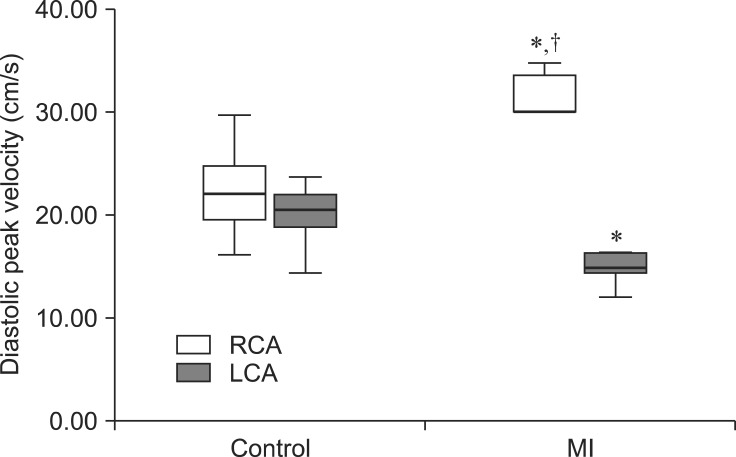 | Fig. 3Box-and-whisker plot of the diastolic peak velocity of normal dogs and model canines with MI. The MI group had significantly different peak diastolic velocities in the RCA and LCA compared to those for the control group (*). The RCA also had a more rapid peak diastolic velocity than that of the LCA in the MI group (†). |
There was strong agreement between the two individual observers who performed the coronary artery measurements. Reliability between the observers for the peak diastolic velocities of the LCA (r = 0.70, p = 0.03) and RCA (r = 0.70, p = 0.03) was significantly high for the control group. Reliability between observers for the peak diastolic velocities of the LCA (r = 0.75, p = 0.03) and RCA (r = 0.87, p = 0.01) in the MI group was also significantly high. For the diastolic deceleration time, reliability between observers of the LCA (r = 0.86, p = 0.02) and RCA (r = 0.94, p < 0.01) was indicative of good agreement in the control group. For the MI group, good inter-observer agreement for the diastolic deceleration times of the LCA (r = 0.94, p < 0.01) and RCA (r = 0.95, p < 0.01) was also observed.
Reproducibility had a trend similar to that of the inter-observer agreement. Reproducibility for the diastolic peak velocities of the LCA (r = 0.98, p < 0.01) and RCA (r = 0.85, p < 0.01) was significantly high in the control group. In the MI group, reproducibility for the diastolic peak velocities of the LCA (r = 0.91, p < 0.01) and RCA (r = 0.96, p < 0.01) was also significant. Reproducibility for the diastolic deceleration time of the LCA (r = 0.88, p < 0.01) and RCA (r = 0.93, p < 0.01) was observed in the control group. Significantly good agreement for diastolic deceleration time of the LCA (r = 0.93, p < 0.01) and RCA (r = 0.92, p < 0.01; Table 2) in the MI group was also found.
Go to : 
Discussion
For more accurate analyses of coronary artery blood profiles and hemodynamic measurements, it is preferable to use Doppler guidewire rather than transthoracic echocardiography given the low-quality images and limited frequency of transducers that are associated with the latter technique [34]. However, recent technological advances in digital imaging along with the introduction of high-frequency transducers have enabled the noninvasive and rapid evaluation of the coronary arteries by transthoracic echocardiography [34]. In the present study, we were able to monitor the canine coronary artery in high-resolution images obtained with color Doppler using high-frequency transducers. Echocardiographic coronary blood profiles were successfully acquired and normal coronary artery flow was easily differentiated from abnormal flow.
Images of the LCA obtained from the right parasternal long-axis left ventricular outflow view and short-axis view at the level of pulmonary artery showed that this artery arose from the left coronary cusp of the aorta. The right parasternal long-axis left ventricular outflow view allowed the coronary artery to be viewed more easily, and enabled the appropriate positioning of the beam angle along the line of blood flow for pulsed-wave Doppler analysis. The LCA was detectable when the transducer was rotated slightly clockwise in the right parasternal left ventricular outflow view to observe the left myocardium. This vessel was found to have an infundibular shape adjacent to the sinus of Valsalva and a thin hypoechoic tubular structure that split to form the paraconal branch. When the beam was inclined clockwise in the left cranial parasternal short-axis view of the aorta to observe the right myocardium, the RCA appeared as a thin tubular structure located at the 12 o'clock position relative to the aorta.
Blood flow velocity in the coronary artery measured using pulsed-wave Doppler analysis was similar to that described in previous studies using Doppler guidewire or intracardiac echocardiography [30]. Pulsed-wave Doppler analysis demonstrated the presence of S- and D-waves in the coronary artery. The S-wave of the systolic phase had a symmetrical waveform with a relatively short deceleration time and low peak velocity. The D-wave of the diastolic phase had a high acceleration time as well as a relatively slow and steady deceleration time and shoulder point. Deceleration times and peak velocities of the D- and S-waves were different.
In animals with cardiomyopathy [7,9,12,19,32], stenotic disease [4,11,14,23], myxomatous mitral valve disease [8,10,21,28,29], cardiovascular tumors [2], or dynamic changes in coronary flow may be associated with coronary arteriosclerosis and MI. A retrospective study on death caused by heart disease in Swedish dogs reported that 32 cases might be cardiomyopathy which was represented dilated cardiomyopathy or myocardial infarction secondary to arteriosclerosis of coronary artery or thromboembolism [9]. Previous studies demonstrated that feline cardiomyopathy presents as diffuse or segmental left ventricular hypertrophy that induces regional systolic dysfunction with myocardial fibrosis and matrix connective tissue accumulation accompanied by moderate or severe intramural coronary arteriosclerosis observed during necropsy [12,19]. In another study about feline hypertrophic cardiomyopathy, it was presumed that this coronary arterial condition could result in a reduction of coronary blood flow [7]. In cases of dilated cardiomyopathy, left ventricular fibrosis and intimal hyperplasia of the intramural coronary arteries also appear with thin and wavy myofibers in young Doberman pinschers with dilated myocardiopathy [32]. The thin and wavy myofibers are associated with changes of oxygen-dependent processes in myocytes as well as myocardial ischemia in humans and dogs [32]. Reverse systolic flow from the isometric ventricular contraction to the aortic ejection, concentric left ventricular hypertrophy, intramural thickening of the coronary arteries, and degeneration of left the ventricular myocardium have been found in dogs with subaortic stenosis [23]. Additionally, a relationship between intramural coronary atherosclerosis and left ventricular hypertrophy was reported in 49 dogs with subaortic stenosis, demonstrating that coronary atherosclerosis may be caused by excessive force generation secondary to left ventricular hypertrophy [11]. A case of left coronary aneurysmal dilation in a dog with subaortic stenosis was also described [14].
In the present study, the exact cause of LCA aneurysmal dilation was uncertain although elevated coronary vascular resistance secondary to left ventricular hypertrophy might have contributed to LCA dilation with poststenotic aortic outflow directed toward the LCA [14]. A previous study reported that English bulldogs with pulmonic stenosis showed congenital coronary artery anomaly of single RCA and abnormal circumpulmonary LCA [4]. Furthermore, myocardial infarction of the left ventricular wall was occurred in these dogs [4]. Causes of myocardial infarction in the left ventricle were not clarified in our investigation, but compression of the intramyocardial segment of the LCA is thought to be associated with hemodynamic changes at parturition [4]. In a previous study, proliferative changes and hyaline plaque formation were observed in dogs with myxomatous mitral valve disease [10]. These changes made the lumen of the coronary arteries narrower. Microscopic intramural MI is a common histopathologic finding in the heart of humans and canines with mitral valve disease [28]. Many factors promote intramural MI in dogs with mitral valve disease. Endothelial dysfunction of the coronary artery and microthrombosis due to platelet aggregation are the most common factors. Previous studies on dogs with mitral valve disease showed that nitric oxide availability decreases in vessel endothelial cells with mitral regurgitation [8,21]. This results in vascular endothelial dilation impairment in the coronary artery, elevated vascular resistance, and intramural myocardial infarction secondary to insufficient oxygen delivery to the myocardium [8,21]. Platelet aggregation is commonly found in dogs with mitral regurgitant that also develop MI due to microthrombosis and perivascular fibrosis of the coronary artery which induces high vascular resistance [28,29]. Previously, a dog with aortic body carcinoma was reported with a mass located on the caudal left atrial epicardial surface that compressed the circumflex branch of the LCA [2]. The presence of MI was confirmed in the region that received blood from the circumflex branch of the LCA [2].
Changes in coronary blood flow are not only observed in cases of cardiovascular diseases such as hypertrophic cardiomyopathy [7,9,12,32] and mitral regurgitation [5,8,10,21,28,29], but also other pathologic conditions such as acute renal failure [18], chronic diabetes mellitus [13,15,31], and hypothyroidism [15,26]. In a previous study, dogs with acute renal failure had decreased or absent coronary flow reserve [18]. These coronary arterial flow changes were affected to dysfunction of coronary endothelium about vasodilation. The risk of myocardial infarction is increased if elevated oxygen demand of the myocardium is not satisfied by the coronary blood supply due to endotherial dysfunction of the coronary artery [18]. A previous study reported that 20 out of 30 dogs with diabetes mellitus or hypothyroidism developed atherosclerosis in the coronary artery [15]. Another investigation found that coronary blood flow in dogs with hypothyroidism was reduced compared to that in normal dogs [26]. Additionally, the coronary artery in cases of diabetes mellitus is not sufficiently dilated during exercise so coronary blood flow is less decreased [31] although this effect was not observe in subjects at rest [13].
In MI dogs with LCAs completely ligated below the first diagonal branch, blood flow in the RCA had a rapid diastolic deceleration time compared to the control animals. This was due to the supposed sympathetic reflex that is associated with MI and results in vascular constriction of the RCA, thereby increasing coronary pressure and flow [16,27]. The low diastolic peak velocity and slow deceleration time of blood flow in the LCA suggests that flow restriction is caused by total occlusion of the paraconal branch. Diastolic peak velocity differences between the control and MI groups were significant. This could have been due to collateral circulation from the paraconal branch and posterior descending artery [6]. In the MI group, blood flow velocity differences between the RCA and LCA were significant owing to higher RCA peak velocity values and lower LCA peak velocity values compared to those of normal dogs.
One limitation of this study is that we were unable to acquire images of the distal LCA or monitor S-waves. This was due to mitral inflow and myocardial interference. In addition, only beagles were used as a model of MI. For future studies, it would be beneficial to use animal models based on diverse species with different cardiovascular disease such as cats with hypertrophic cardiomyopathy.
In summary, transthoracic echocardiography enhanced coronary artery blood flow imaging with color Doppler. This helped detect differences in blood flow velocity and pulsed-wave Doppler patterns between dogs with MI and normal canines. Therefore, pulsed-wave Doppler examination of coronary arterial blood flow can be performed to evaluate hemodynamic function if echocardiographic equipment is available. In addition, application of this technique could be extended to evaluating myocardial blood flow in cases of other cardiovascular diseases.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download