Abstract
To investigate 1α,25-(OH)2D3 regulation of matrix metalloproteinase-9 (MMP-9) protein expression during osteoclast formation and differentiation, receptor activator of nuclear factor κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF) were administered to induce the differentiation of RAW264.7 cells into osteoclasts. The cells were incubated with different concentrations of 1α,25-(OH)2D3 during culturing, and cell proliferation was measured using the methylthiazol tetrazolium method. Osteoclast formation was confirmed using tartrate-resistant acid phosphatase (TRAP) staining and assessing bone lacunar resorption. MMP-9 protein expression levels were measured with Western blotting. We showed that 1α,25-(OH)2D3 inhibited RAW264.7 cell proliferation induced by RANKL and M-CSF, increased the numbers of TRAP-positive osteoclasts and their nuclei, enhanced osteoclast bone resorption, and promoted MMP-9 protein expression in a concentration-dependent manner. These findings indicate that 1α,25-(OH)2D3 administered at a physiological relevant concentration promoted osteoclast formation and could regulate osteoclast bone metabolism by increasing MMP-9 protein expression during osteoclast differentiation.
As the sole cell responsible for bone resorption, the osteoclast plays a crucial role in physiological and pathological processes in bone tissue. However, osteoclasts, highly differentiated terminal cells that exist in relatively small numbers, cannot be differentiated by subculturing and have a limited survival time [4]. Thus, restricted in vitro culturing of osteoclasts has hampered in-depth study of these cells. Receptor activator of nuclear factor κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF), two factors that regulate osteoclast formation, enable in vitro culturing of osteoclasts [36]. Additionally, RNAKL and M-CSF have been used to induce the differentiation of in vitro cultured spleen, peripheral blood mononuclear, and RAW264.7 cells into large populations of osteoclasts with high purity, thereby establishing a foundation for the study of osteoclast formation and activation mechanisms at the molecular level [35,37].
Matrix metalloproteinase (MMP) belongs to the zinc-binding endopeptidase enzyme family and is essential for extracellular matrix degradation in a variety of organs including bone. In particular, high expression of MMP-9 levels by osteoclasts plays an important role in extracellular matrix degradation by this type of cell [11,15]. Previous studies have shown that RANKL can increase MMP-9 expression in a concentration-dependent manner while addition of the MMP-9 inhibitor GM6001 inhibits RANKL-induced formation of multinucleated osteoclasts in a dose-dependent manner [11,15,38]. Other recent investigations have shown that osteoclast precursor cells and osteoclasts can express the vitamin D receptor (VDR) [19]. When 1α,25-(OH)2D3 is present above a threshold concentration, it promotes the expression of VDR, and enhances the binding of RANK and RANKL to the osteoclast surface, thus initiating osteoclast differentiation [6]. However, it is unclear whether vitamin D can regulate MMP-9 protein expression during osteoclast formation and activation. In the present study, RANKL and M-CSF were administered in combination to induce the differentiation of osteoclast precursor RAW264.7 cells into osteoclasts as previously reported [12,15]. Different concentrations of 1α,25-(OH)2D3 were used to investigate the effect of this compound on osteoclast morphology, bone resorption, and MMP-9 protein expression levels. Results from this investigation provided insight into the mechanisms underlying vitamin D regulation of osteoclast differentiation and activation, and provided a theoretical basis for the prevention and treatment of bone metabolic diseases.
Murine monocytic RAW 264.7 cells were purchased from the American Type Culture Collection (USA) and cultured as previously described with slight modification [29]. Briefly, RAW264.7 cells were gently re-suspended in Dulbecco's modified Eagle medium (DMEM; Gibco, USA) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific, USA), 4 mM L-glutamine (AMRESCO, USA), 100 IU/mL penicillin (ShanDong LuKang Pharmaceutical Group, China), and 100 mg/L streptomycin (ShanDong LuKang Pharmaceutical Group). The cells were incubated at 37℃ in a humid 5% CO2 atmosphere (Thermo). The medium was replenished after 24 h, and then the recovered cells were further cultured for 24 h. When the cells covered 80~90% of the flask (Corning, USA) sidewall, they were detached from the flask as follows. The cell culture medium was removed, the cells were washed three times with phosphate-buffered saline (PBS), 1 mL of 0.25% trypsin (AMRESCO) was added for 1 min, the flask was tapped to loosen the cells, and the cells in one flask were transferred to four new flasks equally. After four passages, the cells were incubated with one of the following combinations of cytokines for 48 h, 5 days and 9 days: group A: RAW264.7 cells without any cytokines; group B: 30 µg/L RANKL (PeproTech, USA) and 25 µg/L M-CSF (PeproTech); group C: 30 µg/L RANKL, 25 µg/L M-CSF, and 10-10 M 1α,25-(OH)2D3 (Sigma, USA); group D: 30 µg/L RANKL, 25 µg/L M-CSF, and 10-9 M 1α,25-(OH)2D3; and group E: 30 µg/L RANKL, 25 µg/L M-CSF, and 10-8 M 1α,25-(OH)2D3.
RAW264.7 cells were cultured, trypsinized, and centrifuged at 200 × g for 5 min at room temperature. The supernatant was discarded, the cells were re-suspended in alpha modified Eagle medium (α-MEM; Gibco) containing 10% FBS, 4 mM L-glutamine, 100 IU/mL penicillin, and 100 mg/L streptomycin, and seeded in 96-well cell-culture plates (Corning) at a density of 3 × 103 cells/well. Once the cells had adhered to the well walls, the medium was changed to FBS-free α-MEM and the cells were cultured at 37℃ overnight. Cells were then cultured at 37℃ with one of the cytokine combinations (groups A~E) for 48 h. The culture medium was discarded, 200 µL 0.5 g/L MTT (Amresco) was added to each well, and the plate was incubated at 37℃ for 4 h. The supernatant was subsequently discarded, 150 µL dimethyl sulfoxide (DMSO; Shanghai Shenggong Biological Engineering, China) per well was added with slight shaking for 10 min, and the optical density at 490 nm (OD490) of each well was measured using a Sunrise-basic microplate reader (Tecan, Australia).
RAW264.7 cells cultured as described above were transferred to a 24-well plate (Corning) at a density of 3 × 104 cells/well and cultured at 37℃ for 5 days with one of the cytokine combinations (groups A~E). The cells were then washed with PBS, fixed at room temperature for 10 min with 4% paraformaldehyde, stained using a TRAP staining kit (Sigma, USA) for 1 h at 37℃. The cells were examined with a TE 2000-U inverted microscope (Nikon, Japan) at 200× magnification and images were recorded. Osteoclasts were defined as TRAP-positive multinucleated cells (≥ three nuclei).
One sterile bovine cortical bone slice (50-µm thick) (Peking University First Hospital, China) was placed in each well of a 48-well plate (Corning). Next, RAW264.7 cells cultured as described above were transferred to the bone slice at a density of 1 × 104 cells/well. The cells were cultured with one of the cytokine combinations (groups A~E) for 9 days and the bone slices were then removed. The bone slices were washed three times with PBS, sonicated three times for 5 min in 0.25 M ammonium hydroxide with a KQ-250 ultrasonic cleaner (Kunshan Ultrasonic Instrument, China), and cells attached to the bone slice were removed to expose the lacunae. After being dehydrated in a graded ethanol series (v/v) of 40%, 70%, 80%, 95%, and 100%, and air-dried, the bone slices were gilded using an ion-plating apparatus SCD500 Sputter Coater (Bal-Tec, Liechtenstein) and viewed with an XL30-ESEM environmental scanning electron microscope (Philips, The Netherlands). The area of lacunar resorption was measured by JD801 image analysis (Jiangsu JEDA Science-Technology Development, China).
RAW264.7 cells were transferred to a 6-well plate (Corning) at a density of 1 × 106 cells/well and incubated at 37℃ with one of the cytokine combinations (groups A~E) for 48 h. The cells were washed twice with cold PBS. The fixed cells were lysed in Radio-Immunoprecipitation assay (RIPA; Sigma, USA) buffer on ice for 30 min, and sonicated on ice with ultrasonics processors VCX-1300 (Sonics & Materials, USA) for 3 min. The cell lysates were centrifuged at 12,000 × g for 10 min at 4℃. The protein concentration was measured using an Enhanced BCA Protein Assay Kit (Beyotime Institute of Biotechnology, China). Equal amounts of protein were diluted with sodium dodecyl sulfate (SDS) sample buffer, boiled for 10 min at 100℃, and electrophoresis performed using SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Samples (50 µg) were loaded in each lane and separated at a constant voltage of 110 V for 90 min. The gel was cut and the proteins were transferred to nitrocellulose membranes (Poll, USA). The membranes were blocked with 5% nonfat milk in Tris-buffered saline with 0.1% Tween-20 (TBST) at room temperature for 2 h and then incubated overnight at 4℃ with rabbit anti-mouse (mice) MMP-9 antibody (Abcam, UK) or rabbit anti-mouse GAPDH antibody (Cell Signaling Technology, USA) at a 1 : 1000 dilution. After being washed with TBST (five washes for 5 min each), the membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (at a dilution of 1 : 5,000; Cell Signaling Technology) at room temperature for 2 h. After additional washes with TBST, antibody binding was visualized using an ECL detection kit (Thermo) according to the manufacturer's instructions and the blots were exposed to X-ray film (Eastman Kodak Company, USA). Band volume was measured by Molecular Imager Gel Doc XR System (Bio-Rad, USA) and normalized to bands corresponding to GAPDH.
To evaluate the effects of 1α,25-(OH)2D3 on RAW264.7 cell growth, an MTT assay was performed to evaluate cell proliferation. As shown in Fig. 1, the proliferation rate of RAW264.7 cells cultured for 48 h in the presence of RANKL and M-CSF (groups B~E) was significantly higher (p < 0.01) than that of the control group (group A). However, the cell proliferation rate of all three groups incubated with 1α,25-(OH)2D3 in addition to RANKL and M-CSF (groups C~E) was lower than that of the group exposed to RANKL and M-CSF alone (group B; p < 0.05 for group D and p < 0.01 for group E).
After 5 days of culturing, no osteoclasts were found in group A (Fig. 2A). In contrast, TRAP-positive multinucleated osteoclasts formed in all the other groups (Figs. 2B-E). The number of TRAP-positive multinucleated osteoclasts in group B (3.40 ± 1.14 cells/visual field) was significantly larger than that found in the control group (group A: 0 cells/visual field, p < 0.01; Fig. 3). The addition of 1α,25-(OH)2D3 increased the number of osteoclasts that formed (Fig. 3). In the presence of 1α,25-(OH)2D3, the number of TRAP-positive cells increased to 3.60 ± 1.52 (group C: 30 µg/L RANKL, 25 µg/L M-CSF, and 10-10 M 1α,25-(OH)2D3), 5.40 ± 1.14 (group D: 30 µg/L RANKL, 25 µg/L M-CSF, and 10-9 M 1α,25-(OH)2D3), or 6.80 ± 1.92 (group E: 30 µg/L RANKL, 25 µg/L M-CSF, and 10-8 M 1α,25-(OH)2D3) compared to group B (25 µg/L M-CSF and 30 µg/L RANKL: 3.40 ± 1.14 cells/visual field, p < 0.05 or p < 0.01; Fig. 3).
When the RAW264.7 cells were incubated for 9 days with bovine cortical bone slices, bone resorption lacunae were observed in the slices of all groups except for group A (Figs. 4A-E). The area of lacunar resorption (Fig. 5) in the presence of 25 µg/L M-CSF and 30 µg/L RANKL (group B: 3693 ± 681 µm2/visual field) was significantly larger (p < 0.01) than of the control group (group A: 0 µm2/visual field). The addition of 1α,25-(OH)2D3 enlarged the area of lacunar resorption (Fig. 5). In the presence of 1α,25-(OH)2D3 (Fig. 5), the area of lacunar resorption significantly increased (p < 0.05 or p < 0.01) to 4509 ± 532 µm2 (group C: 30 µg/L RANKL, 25 µg/L M-CSF, and 10-10 M 1α,25-(OH)2D3), 5377 ± 600 µm2 (group D: 30 µg/L RANKL, 25 µg/L M-CSF, and 10-9 M 1α,25-(OH)2D3), or 10,308 ± 1,316 µm2 (group E: 30 µg/L RANKL, 25 µg/L M-CSF, and 10-8 M 1α,25-(OH)2D3) compared to group B (25 µg/L M-CSF and 30 µg/L RANKL: 3,693 ± 681 µm2/visual field).
In the Western blot analysis, total cell protein from every group generated positive bands 92 kDa in size (Fig. 6A). MMP-9 protein expression levels in the cells incubated with RANKL and M-CSF were significantly higher than that in the control group (p < 0.01; Fig. 6B). MMP-9 protein expression in group C was not significantly different from that found in group B. In contrast, 1α,25 (OH)2D3 at 10-9 M and 10-8 M induced significantly higher levels of MMP-9 protein expression than those observed in group B (p < 0.05 and < 0.01, respectively; Fig. 6B).
Vitamin D and its active metabolites are important regulators of calcium and phosphorus metabolism in animals, and have an important impact on bone formation and structure. 1α,25-(OH)2D3, the primary active form of vitamin D, increases intestinal calcium and phosphorus absorption, thus promoting bone calcification. This compound also acts directly on bone cells through the VDR or independently [1,20,33].
Osteoclasts are directly or indirectly regulated by a variety of cytokines, and appear as multinucleated cells after proliferation, fusion, and differentiation. RANKL and M-CSF are important stimulatory factors that influence osteoclast formation and differentiation. Competitive binding between RANKL and RANK on the cell membrane of osteoclast precursors induces osteoclast differentiation. On the other hand, M-CSF promotes osteoclast formation and proliferation primarily through binding to the c-Fms receptor on the osteoclast precursor cell surface [5,13]. In the present study, RAW264.7 cells were incubated with RANKL and M-CSF along with different concentrations of 1α,25-(OH)2D3. The results showed that 1α,25-(OH)2D3 inhibited RAW264.7 cell proliferation in a dose-dependent manner.
1α,25-(OH)2D3, a factor in the vitamin D receptor pathway, is a powerful stimulator of bone resorption. This compound promotes RANKL expression in osteoblasts and stromal cells when bound to its receptor, VDR. Binding between RANKL and RANK initiates a differentiation cascade in osteoclast precursor cells, promotes their differentiation into osteoclasts, and increases osteoclast numbers [21]. Recently, it was shown that 1α,25-(OH)2D3 can increase the number of osteoclasts derived from bone marrow stem cells through RANKL and M-CSF in vitro [18,22]. The primary mechanism involved an increase in the RANKL : osteoprotegerin ratio in osteoblasts compared to bone marrow stromal cells that indirectly regulates osteoclast formation and differentiation [3,28]. M-CSF plays an important role in the induction of osteoclast proliferation [9,10,30]. In the present study, TRAP-positive multinucleated osteoclasts were observed after 5 days of incubating RAW264.7 cells in culture medium containing RANKL and M-CSF. The addition of 1α,25-(OH)2D3 to this medium resulted in a dose-dependent increase in the number of osteoclasts that reached a maximum with 10-8 M. This result was similar to data from a study by Vincent et al. [31], and concurs with the finding that 1α,25-(OH)2D3 inhibited RAW264.7 cell proliferation in a dose-dependent manner. Furthermore, 1α,25-(OH)2D3 can inhibit the proliferation of various cells, including human periodontal ligament cells [27] and primary osteoblasts [2], while encouraging cell differentiation [23,34]. After 9 days of incubation in induction medium, significant resorption lacunae were formed on bovine cortical bone slices. Lacunae formation was increased in a dose-dependent manner by 1α,25-(OH)2D3 with the most extensive effect observed with 10-8 M. These results are consistent with those reported by Naruse et al. [22,26], and confirmed that radial bone mineral density (BMD) decreased across increasing concentrations of 1, 25(OH)2D3 [7].
MMP-9 was first discovered in the osteoclast lineage during mouse development by Repone et al. [25]. This proteolytic enzyme is highly expressed in osteoclasts and important for extracellular matrix degradation during bone resorption as well as bone remodeling. A number of studies have been conducted to elucidate the regulatory mechanism governing osteoclastic MMP-9 protein expression during bone absorption [14,16,32].
RAW264.7 cells are osteoclast precursors and can express phenotype marker genes characteristic of mature osteoclasts [8]. It has been shown that the osteolytic functions of osteoclasts are mainly facilitated by the secretion of carbonic anhydrase II, MMP-9, and other enzymes into closed cavities formed on the bone surface [24]. In the present study, different concentrations of 1α,25-(OH)2D3 were added to induce the differentiation of RAW264.7 cells into osteoclasts after incubation with RANKL and M-CSF. The capacity of 1α,25-(OH)2D3 to enhance monocyte fusion for the formation of osteoclasts was increased in a dose-dependent manner. At 10-8 M, 1α,25-(OH)2D3 significantly increased the number of osteoclasts, the bone resorption capacity of the osteoclasts, and MMP-9 protein expression. These observations were similar to those reported by Kido [17].
In summary, 1α,25-(OH)2D3 was found to increase the number of osteoclasts cultured in vitro in a dose-dependent manner. MMP-9 protein expression was also elevated and bone resorption was enhanced. Our findings demonstrate that 1α,25-(OH)2D3 plays a crucial role in bone metabolism.
Figures and Tables
 | Fig. 1MTT analysis of RAW264.7 cell viability after treatment with 1α,25-(OH)2D3, receptor activator of nuclear factor κB ligand (RANKL), and macrophage colony-stimulating factor (M-CSF). The results are expressed as the mean ± standard error (SE). RANKL (30 µg/L) plus M-CSF (25 µg/L) promoted the proliferation of RAW264.7 cell (**p < 0.01 vs. group A). Additionally, 1α,25-(OH)2D3 inhibited the proliferation rate of RAW264.7 cells in a dose-dependent manner (†p < 0.05 vs. group B, ††p < 0.01 vs. group B). |
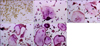 | Fig. 2Morphology and number of TRAP-positive osteoclasts in RAW264.7 cells after 5 day of incubation with or without cytokines. No osteoclast formation was observed in group A (RAW264.7 cells cultured without any cytokines). In contrast, many osteoclasts (indicated by black arrows) were formed in groups B ~ E. (A) No cytokines. (B) 30 µg/L RANKL and 25 µg/L M-CSF. (C) 30 µg/L RANKL, 25 µg/L M-CSF, and 10-10 mol/L 1α, 25-(OH)2D3. (D) 30 µg/L RANKL, 25 µg/L M-CSF, and 10-9 mol/L 1α, 25-(OH)2D3. (E) 30 µg/L RANKL, 25 µg/L M-CSF, and 10-8 mol/L 1α, 25-(OH)2D3. Scale bars = 100 µm. |
 | Fig. 3The number of TRAP-positive multinucleated osteoclasts formed with or without M-CSF, RANKL, and 1α,25-(OH)2D3. No osteoclasts were formed in group A (RAW264.7 cells cultured without any cytokines). M-CSF and RANKL induced the formation of TRAP-positive multinucleated osteoclasts (groups B~E). In addition, 1α,25-(OH)2D3 increased the number of TRAP-positive multinucleated osteoclasts (groups C ~E). **p < 0.01 vs. group A, †p < 0.05 vs. group B, and ††p < 0.01 vs. group B. |
 | Fig. 4Resorption lacunae on bone slices observed with an XL30-ESEM environmental scanning electron microscope. No resorption lacunae were observed on bone slices incubated with cells from group A (RAW264.7 cells cultured without any cytokines). In contrast, many resorption lacunae (indicated with white arrows) were formed in bone slices incubated with cells from groups B ~ E. (A) No cytokines. (B) 30 µg/L RANKL and 25 µg/L M-CSF. (C) 30 µg/L RANKL, 25 µg/L M-CSF, and 10-10 mol/L 1α, 25-(OH)2D3. (D) 30 µg/L RANKL, 25 µg/L M-CSF, and 10-9 mol/L 1α, 25-(OH)2D3. (E) 30 µg/L RANKL, 25 µg/L M-CSF, and 10-8 mol/L 1α, 25-(OH)2D3. Scale bars = 50 µm. |
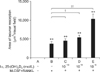 | Fig. 5The area of lacunar resorption. No resorption lacunae were observed in bone slices incubated with cells from group A (RAW264.7 cells cultured without any cytokines). M-CSF and RANKL (group B) induced osteoclast bone resorption. Additionally, 1α,25-(OH)2D3 enhanced osteoclast bone resorption (groups C ~ E). **p < 0.01 vs. group A, †p < 0.05 vs. group B, and ††p < 0.01 vs. group B. |
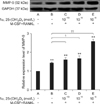 | Fig. 6Detection of MMP-9 protein expression in osteoclasts by Western blotting. The results are expressed as the mean ± SE. MMP-9 was expressed in each group of cells. Compared to group A (RAW264.7 cells cultured without any cytokines), RANKL and M-CSF enhanced the expression of MMP-9 (**p < 0.01 vs. control group). Furthermore, 1α,25-(OH)2D3 promoted the expression of MMP-9 in a dose-dependent manner (†p < 0.05 vs. group B and ††p < 0.01 vs. group B). |
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 31172373 and 31372495), the National Science Foundation for Distinguished Young Scholars of China (Grant No. 31302154), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (Grant No. 13KJB230002), Specialized Research Fund for the Doctoral Program of Higher Education (Grant No. 20133250120002) and the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). In addition, the authors would like to thank the Testing Center of Yangzhou University for providing technical support for the ESEM conducted this project.
References
1. Anderson PH, Lam NN, Turner AG, Davey RA, Kogawa M, Atkins GJ, Morris HA. The pleiotropic effects of vitamin D in bone. J Steroid Biochem Mol Biol. 2013; 136:190–194.

2. Atkins GJ, Anderson PH, Findlay DM, Welldon KJ, Vincent C, Zannettino ACW, O'Loughlin PD, Morris HA. Metabolism of vitamin D3 in human osteoblasts: Evidence for autocrine and paracrine activities of 1α,25-dihydroxyvitamin D3. Bone. 2007; 40:1517–1528.

3. Bergh JJ, Xu Y, Farach-carson MC. Osteoprotegerin expression and secretion are regulated by calcium influx through the L-type voltage-sensitive calcium channel. Endocrinology. 2004; 145:426–436.

4. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003; 423:337–342.

5. Brooks PJ, Heckler AF, Wei K, Gong SG. M-CSF accelerates orthodontic tooth movement by targeting preosteoclasts in mice. Angle Orthod. 2011; 81:277–283.

6. Brown AJ, Zhong M, Finch J, Ritter C, Slatopolsky E. The roles of calcium and 1,25-dihydroxyvitamin D3 in the regulation of vitamin D receptor expression by rat parathyroid glands. Endocrinology. 1995; 136:1419–1425.

7. Christensen MHE, Apalset EM, Nordbø Y, Varhaug JE, Mellgren G, Lien EA. 1,25-dihydroxyvitamin D and the vitamin D receptor gene polymorphism Apa1 influence bone mineral density in primary hyperparathyroidism. PLoS One. 2013; 8:e56019.

8. Cuetara BLV, Crotti TN, O'Donoghue AJ, McHugh KP. Cloning and characterization of ostoeclast precursors from the RAW264.7 cell line. In Vitro Cell Dev Biol Anim. 2006; 42:182–188.

9. Felix R, Cecchini MG, Fleisch H. Macrophage colony stimulating factor restores in vivo bone resorption in the op/op osteopetrotic mouse. Endocrinology. 1990; 127:2592–2594.

10. Fujikawa Y, Sabokbar A, Neale SD, Itonaqa I, Torisu T, Athanasou NA. The effect of macrophage-colony stimulating factor and other humoral factors (interleukin-1, -3, -6, and -11, tumor necrosis factor-α, and granulocyte macrophage-colony stimulating factor) on human osteoclast formation from circulating cells. Bone. 2001; 28:261–267.

11. Fujisaki K, Tanabe N, Suzuki N, Kawato T, Takeichi O, Tsuzukibashi O, Makimura M, Ito K, Maeno M. Receptor activator of NF-κB ligand induces the expression of carbonic anhydrase II, cathepsin K, and matrix metalloproteinase-9 in osteoclast precursor RAW264.7 cells. Life Sci. 2007; 80:1311–1318.

12. Fu YX, Gu JH, Zhang YR, Tong XS, Zhao HY, Yuan Y, Liu XZ, Bian JC, Liu ZP. Osteoprotegerin influences the bone resorption activity of osteoclasts. Int J Mol Med. 2013; 31:1411–1417.

13. Hodge JM, Kirkland MA, Nicholson GC. Multiple roles of M-CSF in human osteoclastgenesis. J Cell Biochem. 2007; 102:759–768.
14. Inui T, Ishibashi O, Origane Y, Fujimori K, Kokubo T, Nakajima M. Matrix metalloproteinases and lysosomal cysteine proteases in osteoclasts contribute to bone resorption through distinct modes of action. Biochem Biophys Res Commun. 1999; 258:173–178.

15. Kaneshita Y, Goda S, Kawamoto T. The effect of matrix metalloproteinase-9 on the differentiation into osteoclast cells on RAW264 cells. Orthod Waves. 2007; 66:122–128.

16. Kawatani M, Osada H. Osteoclast-targeting small molecules for the treatment of neoplastic bone metastases. Cancer Sci. 2009; 100:1999–2005.

17. Kido S, Inoue D, Hiura K, Javier W, Ito Y, Matsumoto T. Expression of RANK is dependent upon differentiation into the macrophage/osteoclast lineage: induction by 1α,25-dihydroxyvitamin D3 and TPA in a human myelomonocytic cell line, HL60. Bone. 2003; 32:621–629.

18. Kudo O, Sabokbar A, Pocock A, Itonaga I, Athanasou NA. Isolation of human osteoclasts formed in vitro: hormonal effects on the bone-resorbing activity of human osteoclasts. Calcif Tissue Int. 2002; 71:539–546.

19. Langub MC, Reinhardt TA, Horst RL, Malluche HH, Koszewski NJ. Characterization of vitamin D receptor immunoreactivity in human bone cells. Bone. 2000; 27:383–387.

20. Lieben L, Carmeliet G. Vitamin D signaling in osteocytes: effects on bone and mineral homeostasis. Bone. 2013; 54:237–243.

21. Matsuzaki K, Udagawa N, Takahashi N, Yamaguchi K, Yasuda H, Shima N, Morinaga T, Toyama Y, Yabe Y, Higashio K, Suda T. Osteoclast differentiation factor (ODF) induces osteoclast-like cell formation in human peripheral blood mononuclear cell cultures. Biochem Biophys Res Commun. 1998; 246:199–204.

22. Naruse M, Otsuka E, Naruse M, Ishihara Y, Miyagawa-Tomita S, Hagiwara H. Inhibition of osteoclast formation by 3-methylcholanthrene, a ligand for arylhydrocarbon receptor: suppression of osteoclast differentiation factor in osteogenic cells. Biochem Pharmacol. 2004; 67:119–127.

23. Piek E, Sleumer LS, van Someren EP, Heuver L, de Haan JR, de Grijs I, Gilissen C, Hendriks JM, van Ravestein-van Os RI, Bauerschmidt S, Dechering KJ, van Zoelen EJ. Osteo-transcriptomics of human mesenchymal stem cells: ccelerated gene expression and osteoblast differentiation induced by vitamin D reveals c-MYC as an enhancer of BMP2-induced osteogenesis. Bone. 2010; 46:613–627.

24. Reynolds JJ. Collagenases and tissue inhibitors of metalloproteinases: a functional balance in tissue degradation. Oral Dis. 1996; 2:70–76.

25. Reponen P, Sahlberg C, Munaut C, Thesleff I, Tryggvason K. High expression of 92-kD type IV collagenase (Gelatinase B) in the osteoclast lineage during mouse development. J Cell Biol. 1994; 124:1091–1102.

26. Skjødt H, Gallagher JA, Beresford JN, Couch M, Poser JW, Russell RGG. Vitamin D metabolites regulate osteocalcin synthesis and proliferation of human bone cell in vitro. J Endocrinol. 1985; 105:391–396.

27. Tang X, Meng H. Osteogenic induction and 1,25-dihydroxyvitamin D3 oppositely regulate the proliferation and expression of RANKL and the vitamin D receptor of human periodontal ligament cells. Arch Oral Biol. 2009; 54:625–633.

28. Thomas GP, Baker SUK, Eisman JA, Gardiner EM. Changing RANKL/OPG mRNA expression in differentiating murine primary osteoblasts. J Endocrinol. 2001; 170:451–460.

29. Ueda Y, Kanayama M, Yamauchi N, Iio C, Taira Z. Effects of Hachimi-jio-gan extract on intestinal absorption of calcium in ovariectomized mice and stimulation of RANKL-induced osteoclast differentiation of Raw264.7 cells by lipopolysaccharide. J Hard Tissue Biol. 2012; 21:469–476.

30. Van Wesenbeeck L, Odgren PR, MacKay CA, D'Angelo M, Safadi FF, Popoff SN, Van Hul W, Marks SC Jr. The osteopetrotic mutation toothless (tl) is a loss-of-function frameshift mutation in the rat Csf1 gene: evidence of a crucial role for CSF-1 in osteoclastogenesis and endochondral ossification. Proc Natl Acad Sci U S A. 2002; 99:14303–14308.

31. Vincent C, Kogawa M, Findlay DM, Atkins GJ. The generation of osteoclasts from RAW 264.7 precursors in defined, serum-free conditions. J Bone Miner Metab. 2009; 27:114–119.

32. de Vrieze E, Sharif F, Metz JR, Flik G, Richardson MK. Matrix metalloproteinases in osteoclasts of ontogenetic and regenerating zebrafish scales. Bone. 2011; 48:704–712.

33. Willems HME, van den Heuvel EGHM, Carmeliet G, Schaafsma A, Klein-Nulend J, Bakker AD. VDR dependent and independent effects of 1,25-dihydroxyvitamin D3 on nitric oxide production by osteoblasts. Steroids. 2012; 77:126–131.

34. Woods C, Domenget C, Solari F, Gandrillon O, Lazarides E, Jurdic P. Antagonistic role of vitamin D3 and retinoic acid on the differentiation of chicken hematopoietic macrophages into osteoclast precursor cells. Endocrinology. 1995; 136:85–95.

35. Yao Z, Xing L, Qin C, Schwarz EM, Boyce BF. Osteoclast precursor interaction with bone matrix induces osteoclast formation directly by an interleukin-1-mediated autocrine mechanism. J Biol Chem. 2008; 283:9917–9924.

36. Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998; 95:3597–3602.

37. Yavropoulou MP, Yovos JG. Osteoclastogenesis - current knowledge and future perspectives. J Musculoskelet Neuronal Interact. 2008; 8:204–216.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download