Abstract
IS901 RFLP analysis of 36 Mycobacterium avium subsp. avium (MAA) isolates from 15 pheasants (Phasianus colchicus) and two goshawks (Accipiter gentilis) from four pheasant farms was performed. Using this method, six different IS901 RFLP types (E, F, G, M, Q, and V) were identified. The distribution of IS901 RFLP profiles was tightly linked to individual flocks. Matching IS901 RFLP profiles observed in the present study indicate MAA transmission between pheasants and goshawks in the same locality. In two flocks, different pheasants within a flock as well as in various organs of five individual pheasants were found to have two distinct IS901 RFLP profiles.
Avian tuberculosis caused by Mycobacterium avium subsp. avium (MAA), a member of the Mycobacterium avium complex (MAC), is a disease that affects many different animal species worldwide such as pigs, cattle, and particularly birds which are considered to be the main reservoir for this organism [13]. The spread of MAA from birds to other susceptible animal species occurs primarily through fecal contamination of the environment. Individual animals are subsequently infected through the ingestion or inhalation of contaminated food, water, or soil particles [4,14]. Clinically, avian tuberculosis in birds manifests itself as a chronic wasting disease. However, many cases never show signs of infection. The disease is diagnosed upon post-mortem examination when tuberculous lesions are found during gross examination or granulomatous inflammation is discovered during histopathological examination [12,14].
Molecular studies together with anamnestic data can help improve our understanding of avian tuberculosis epidemiology among birds. Several molecular approaches e.g., pulsed field gel electrophoresis (PFGE), random amplification of polymorphic DNA (RAPD), amplified fragment length polymorphism (AFLP), mycobacterial interspersed repetitive unit-variable number of tandem repeat (MIRU-VNTR), and IS901 restriction fragment length polymorphism (RFLP) have been used to study of MAA isolates [5,8,9,11]. Currently, there is an increasing trend in the number of groups using MIRU-VNTR analysis for typing Mycobacterium tuberculosis isolates. Unfortunately, the results of MIRU-VNTR analysis of MAA isolates reported by Pate et al. [9] show that this technique is not sufficiently discriminatory. RAPD has been found to have discriminatory power appropriate for the differentiation of MAA isolates [11], but a major disadvantage of this method is the poor reproducibility of results between different labs. In contrast, IS901 RFLP data, due to the simple IS901 RFLP profile that consists of about 14 bands, are reproducible between labs [1].
Only a few studies [2,11,12] on the etiology of MAA infection have been conducted. In order to gain new information regarding avian tuberculosis, the genetic variability of MAA isolates from captive pheasants in four different flocks and two free living goshawks was evaluated. The 34 MAA isolates used in this study were recovered from tissues of 15 pheasants originating from four distinct farms (A, B, C, and D) in the Czech Republic. Additionally, two MAA isolates detected in the livers of two dead goshawks (Accipiter gentilis) found in the vicinity of farm A were also included in our analysis. Anamnestic data for the 14 pheasants and two goshawks are presented in our previous study [7]. Isolates from other organ tissues such as the lungs, kidneys, and ovaries of the pheasants were also included in the study. Additionally, three MAA isolates from one pheasant with hepatic lesions were collected at farm C and used for typing. The cultivation of different tissues on solid media (Herrold's egg yolk and Stonebrink's media) and PCR identification of MAA were carried out as previously described by Fischer et al. [3] and Moravkova et al. [6]. IS901 RFLP was conducted with the restriction enzyme PvuII (New England Biolabs, USA) and data analysis was carried out according to a standardized protocol [1].
A total of six IS901 RFLP profiles, E, F, G, M, Q, and V (Fig. 1), were found among 34 MAA isolates obtained from 15 pheasants and two goshawks. All IS901 RFLP profiles we generated were previously described by Dvorska et al. [1]. The distribution of IS901 RFLP profiles was strictly limited to individual flocks. For two flocks (A and B), two distinct IS901 RFLP profiles were found in different pheasants within the flocks as well as in various organs of five individual pheasants (Table 1). In all cases, the IS901 RFLP patterns only varied by the position of one band. This could have been due to a mutation in the mycobacterial genome or exposure of the birds to multiple mycobacterial sources. Similar results were also obtained in our previous studies in which two different IS901 RFLP profiles were detected in 33.3% of pig farms [5] and one poultry flock in which polyclonal infection was suspected [12].
Birds are considered to be sensitive to MAA but disease progression varies among individual birds depending upon immune status, stress levels, avian taxon, and strain virulence [2,11]. It has been documented that MAA virulence is multifactorial and IS901 may play an important role [10]. In our previous study of avian tuberculosis in waterfowl [2], it was found that all infected birds had MAA with an IS901 RFLP profile F whereas two other IS901 RFLP profiles (G and T) were only isolated from the feces of one bird. Subsequent testing revealed that the G and T isolates are not virulent to pullets in contrast to those with F profiles that are fully virulent. In the present study, granulomatous lesions observed in five MAA-positive pheasants (nos. 1, 2, 9, 11, and 14) had four IS901 RFLP types (G, F, M, and V). No granulomatous lesions in the parenchymatous organs were observed during the post-mortem examination of the two goshawks with IS901 RFLP type G (Table 1). Shitaye et al. [12] studied the RFLP profiles of a flock of MAA-infected hens. Isolates recovered from tissues with and without tuberculous lesions exhibited IS901 RFLP types AE and E. IS901 RFLP type AD was isolated from tissues lacking pathological lesions. Therefore, we conclude that the virulence of different strains cannot be assessed by IS901 RFLP typing alone and further studies examining the virulence factors of different strains MAA are necessary.
The identification of identical IS901 RFLP profiles in different animal species may serve as evidence of interspecies transmission or a common source of infection [2,9]. In the current study, we detected MAA infection in pheasants with the same IS901 RFLP profile E (Table 1) previously found in hens on the same farm [12]. The pheasants and hens were kept in separate areas, and direct contact between the two bird populations was prevented. Therefore, we can assume that contaminated shoes and clothes of farm personnel, feed, drinking water, or instruments are likely vectors of MAA. Interestingly, IS901 RFLP profile G, which was only detected in one pheasant flock, was also identified in isolates from the livers of two goshawks from the same locality (Table 1). Since the goshawk is a predator, it is probable that these birds became infected upon consuming prey such as an infected pheasant or other infected animals inhabiting the same locality as the infected pheasants.
In summary, all IS901 RFLP profiles detected in pheasants were detected previously in different animal species [1]. The distribution of IS901 RFLP profiles was tightly linked to the individual flocks. Further, in two flocks two distinct IS901 RFLP profiles of MAA isolates that differ in position of one band were observed. MAA isolates from pheasants and goshawks originated from the same locality displayed identical IS901 RFLP profile which indicate the common source of infection for both or MAA transmission between pheasants and goshawks.
Figures and Tables
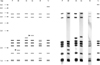 | Fig. 1Six IS901 RFLP profiles of Mycobacterium avium subsp. avium isolates after digestion with restriction endonuclease PvuII. Band sizes of a 1-kbp ladder are shown on the left of the patterns. Arrowheads indicate differences between IS901 RFLP profiles in an individual flock. Flock A: IS901 RFLP profiles G and Q, flock B: IS901 RFLP profiles F and M. |
Table 1
Distribution of IS901 restriction fragment length polymorphism (RFLP) profiles for Mycobacterium avium subsp. avium in different pheasants originating from four flocks (A, B, C, and D)

*Granulomatous lesions were present. RFLP: standardised IS901 RFLP with restriction endonuclease PvuII was carried out as described by Dvorska et al. [1], Ph: pheasant (Phasianus colchicus), Go: goshawk (Accipiter gentilis).
References
1. Dvorska L, Bull TJ, Bartos M, Matlova L, Svastova P, Weston RT, Kintr J, Parmova I, Van Soolingen D, Pavlik I. A standardised restriction fragment length polymorphism (RFLP) method for typing Mycobacterium avium isolates links IS901 with virulence for birds. J Microbiol Methods. 2003. 55:11–27.

2. Dvorska L, Matlova L, Ayele WY, Fischer OA, Amemori T, Weston RT, Alvarez J, Beran V, Moravkova M, Pavlik I. Avian tuberculosis in naturally infected captive water birds of the Ardeideae and Threskiornithidae families studied by serotyping, IS901 RFLP typing, and virulence for poultry. Vet Microbiol. 2007. 119:366–374.

3. Fischer O, Mátlová L, Dvorská L, Švástová P, Bartl J, Melichárek I, Weston RT, Pavlík I. Diptera as vectors of mycobacterial infections in cattle and pigs. Med Vet Entomol. 2001. 15:208–211.

4. Matlova L, Dvorska L, Ayele WY, Bartos M, Amemori T, Pavlik I. Distribution of Mycobacterium avium complex isolates in tissue samples of pigs fed peat naturally contaminated with mycobacteria as a supplement. J Clin Microbiol. 2005. 43:1261–1268.

5. Moravkova M, Bartos M, Dvorska-Bartosova L, Beran V, Parmova I, Ocepek M, Pate M, Pavlik I. Genetic variability of Mycobacterium avium subsp. avium of pig isolates. Vet Med (Praha). 2007. 52:430–436.

6. Moravkova M, Hlozek P, Beran V, Pavlik I, Preziuso S, Cuteri V, Bartos M. Strategy for the detection and differentiation of Mycobacterium avium species in isolates and heavily infected tissues. Res Vet Sci. 2008. 85:257–264.

7. Moravkova M, Lamka J, Kriz P, Pavlik I. The presence of Mycobacterium avium subsp. avium in common pheasants (Phasianus colchicus) living in captivity and in other birds, vertebrates, non-vertebrates and the environment. Vet Med (Praha). 2011. 56:333–343.

8. Möbius P, Lentzsch P, Moser I, Naumann L, Martin G, Köhler H. Comparative macrorestriction and RFLP analysis of Mycobacterium avium subsp. avium and Mycobacterium avium subsp. hominissuis isolates from man, pig, and cattle. Vet Microbiol. 2006. 117:284–291.

9. Pate M, Kušar D, Žolnir-Dovč M, Ocepek M. MIRU-VNTR typing of Mycobacterium avium in animals and humans: heterogeneity of Mycobacterium avium subsp. hominissuis versus homogeneity of Mycobacterium avium subsp. avium strains. Res Vet Sci. 2011. 91:376–381.

10. Pavlik I, Matlova L, Dvorska L, Shitaye JE, Parmova I. Mycobacterial infections in cattle and pigs caused by Mycobacterium avium complex members and atypical mycobacteria in the Czech Republic during 2000-2004. Vet Med (Praha). 2005. 50:281–290.

11. Schrenzel M, Nicolas M, Witte C, Papendick R, Tucker T, Keener L, Sutherland-Smith M, Lamberski N, Orndorff D, Heckard D, Witman P, Mace M, Rimlinger D, Reed S, Rideout B. Molecular epidemiology of Mycobacterium avium subsp. avium and Mycobacterium intracellulare in captive birds. Vet Microbiol. 2008. 126:122–131.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download