Abstract
The use of mesenchymal stem cells (MSCs) has emerged as a potential new treatment for myocardial infarction. However, the poor viability of MSCs after transplantation critically limits the efficacy of this new strategy. The expression of microRNA-210 (miR-210) is induced by hypoxia and is important for cell survival under hypoxic conditions. Hypoxia increases the levels of hypoxia inducible factor-1 (HIF-1) protein and miR-210 in human MSCs (hMSCs). miR-210 positively regulates HIF-1α activity. Furthermore, miR-210 expression is also induced by hypoxia through the regulation of HIF-1α. To investigate the effect of miR-210 on hMSC survival under hypoxic conditions, survival rates along with signaling related to cell survival were evaluated in hMSCs over-expressing miR-210 or ones that lacked HIF-1α expression. Elevated miR-210 expression increased survival rates along with Akt and ERK activity in hMSCs with hypoxia. These data demonstrated that a positive feedback loop involving miR-210 and HIF-1α was important for MSC survival under hypoxic conditions.
Bone marrow-derived mesenchymal stem cells (MSCs) are an excellent model for developing cell-based therapeutics because of their potential for repairing damaged tissue. In particular, MSCs possess advantageous attributes such as ease of isolation, high expansion potential, genetic stability, and multipotent capacity. For these reasons, MSCs have emerged as the most suitable candidate for cellular therapy and tissue engineering techniques. Several preclinical studies have demonstrated that MSCs help prevent deleterious ventricular remodeling and improve the recovery of cardiac performance after myocardial infarction [23,31]. However, Toma et al. [34] reported a very low survival rate (<1%) of MSCs transplanted into ischemic hearts 4 days after transplantation. Poor MSC viability after transplantation critically limits the efficacy of this new therapeutic strategy [23,25,31].
A major challenge to stem cell therapy is that transplanted stem cells undergo apoptosis at an accelerated rate given that they are exposed to an extremely harsh, pro-apoptotic microenvironment in the injured region [10]. In addition to a poor blood supply and low oxygen tension, inflammation triggered by tissue damage may generate oxidative stress as well as cytotoxic radicals and proteins, all of which may cause the death of transplanted cells through several different mechanisms including apoptosis [10]. In particular, hypoxia, defined as an insufficient oxygen supply to tissues, can result from physiological or pathological conditions and changes the physiological characteristics of embryonic and adult stem cells [6]. The mechanism underlying hypoxic condition might be primarily attributed to the hypoxia inducible factor-1 (HIF-1) signal pathway [37]. HIFs control cellular responses to hypoxia by regulating genes that are involved in metabolism, angiogenesis, erythropoiesis, cell proliferation, differentiation, and apoptosis [13]. HIF is a heterodimer consisting of an oxygen-sensitive alpha subunit (HIFα) and a constitutively active beta subunit (HIF-1β; aryl hydrocarbon receptor nuclear translocator). Three isoforms of HIFα have been identified. HIF-1α and HIF-2α are thought to modulate the majority of responses to hypoxia involving HIFα [21]. In the presence of hypoxia, stabilized HIFα binds to HIF-1β, and the resulting complex regulates the expression of downstream genes involved in adaptation to and protection against low oxygen levels [21].
MicroRNA (miRNA) consists of single-stranded noncoding RNA containing 19-23 nucleotides that mechanistically influences a variety of cellular functions via translational inhibition or degradation of target mRNA. Given the diverse roles of miRNA in numerous cellular functions, the role of this molecule in gene regulation associated with hypoxia is not surprising. Recently, miRNA molecules have emerged as a new class of genes regulated by HIF-1α in response to hypoxia. miRNA-199a (miR-199a), miR-17-92 clusters, and miR-20b regulate HIF-1α with hypoxia [19,29,32] whereas the expression of miR-23, miR-24, miR-26, miR-107, miR-210, and miR-373 is induced by HIFs [5,12]. Following the first report of hypoxia affecting miRNA expression by Ivan and colleagues in 2007 [18], a number of studies have demonstrated that miR-210 is the most consistently and predominantly up-regulated miRNA in hypoxic condition. In cancer cells, miR-210 over-expression under normoxic conditions leads to increased HIF-1α levels [13]. Consequently, HIF-1α activity increases, triggering the accumulation of miR-210 as oxygen levels decrease. Increased miR-210 levels suppress the protein expression of glycerol-3-phosphate dehydrogenase 1-like (GPD1L) as a negative regulator of HIF-1α stability and a direct target of miR-210, resulting in increased activity of HIF-1α [14,28].
In the present study, we hypothesized that the positive feedback loop between miR-210 and HIF-1α would improve the survival of human MSCs (hMSCs) under hypoxic conditions. To test this hypothesis, we confirmed the induction of HIF-1α and miR-210 expression in hypoxic hMSCs. We also determined that HIF-1α and miR-210 mutually regulated each other's activities when miR-210 was over-expressed and HIF-1α was down-regulated in MSCs. Moreover, miR-210 overexpression regulated cell survival and modulated HIF-1 activity.
Cobalt (II) chloride hexahydrate (CoCl2), Z-Leu-Leu-Leu-al (MG132), and anti-β-actin antibody were purchased from Sigma-Aldrich (USA). Anti-HIF-1α antibody (H-206) was obtained from Santa Cruz Biotechnology (USA). Anti-phospho-Akt, Akt, anti-phospho-ERK1/2, and ERK1/2 antibodies were obtained from Cell Signaling (USA).
hMSCs (cat. No. PT-2501) were obtained from Lonza (USA). The cells had been harvested from normal human bone marrow and were maintained at 37℃ humidified atmosphere containing 5% CO2 incubator. Culture media composed 10% fetal bovine serum (FBS; Invitrogen, USA) Dulbecco's modified Eagle's medium (DMEM)-low glucose supplemented with and 100 U/mL penicillin (Invitrogen, USA) and 100 µg/mL streptomycin (Invitrogen, USA). Fresh media was added and replaced every 3 or 4 days. We used the hMSCs after 7~10 passages. At these passage numbers, hMSCs maintained a consistent morphology and were positive for various mesenchymal markers such as CD29, 44, 105, and 166 by flow cytometry (data not show).
MSCs were cultured until 80% confluent and were then grown under hypoxic conditions for 12~24 h. The medium used for culturing under hypoxic conditions was serum-free DMEM containing 100 U/mL penicillin and 100 µg/mL streptomycin. The medium was degassed and then exposed to a gas mixture of 5% CO2, 10% H2, and 85% N2. The hypoxic cells were maintained in an airtight humidified hypoxic chamber (Anaerobic Environment; ThermoForma, USA) at 37℃ and continuously gassed with 85% N2. Cobalt chloride (CoCl2; Sigma, USA) was dissolved in PBS and used at a final concentration of 250 µM in serum-free DMEM under normoxic conditions for 24 h.
miRNA was purchased from Genolution Pharmaceuticals (Korea). hMSCs were transfected with the miRNA at a final concentration of 100 nM using siLentFect Lipid reagent (Bio-Rad, USA) in serum free DMEM. miR-210 mimic, chemically optimized double-stranded RNA, or scrambled RNA oligomer was transfected into cells. After transfection for 4 h, the media were replaced in 10% FBS contained DMEM.
MSCs were lysis using 1 mL TRIzol reagent (Sigma, USA). Total RNA was extracted using chloroform (200 µL) and shaked vigorously for 15 sec and incubate at room temperature for 7 min. Next, centrifugation at 12,000 × g, at 4℃ for 15 min, caused three layers to appear, and the transparent upper aqueous phase was collected in a new tube. Each sample next received 500 µL 2-propanol, and inverted for 10 sec was repeated and centrifugation at 12,000 × g, at 4℃ for 10 min. The pellet washed in diethylpyrocarbonate-contained 75% ethanol. Centrifugation at 7,500 × g, at 4℃ for 5 min, followed. Remove all leftover ethanol and pellet dried about 2 min at room temperature. The isolated RNA was resuspended in 30 µL nuclease-free water. The quantity and quality of the RNA were determined by measuring the OD260/280 ratio with a DU 640 spectrophotometer (Eppendorf, Germany). Next, 100 ng the purified total RNA underwent reverse transcription (Taqman MicroRNA Reverse Transcriptase kit; Applied Biosystems, USA) in combination Taqman MicroRNA assays. The threshold cycle (Ct) of each target gene was automatically defined, located in the linear amplification phase of the PCR, and normalized to control U6 (ΔCt value). The relative difference in expression levels of each miRNAs was calculated (DDCt) and is reported as fold induction (2-ΔΔCt). Amplification and detection of miR-210 or U6 were performed using a TaqMan Small RNA Assay kit (Applied Biosystems, USA) at 95℃ for 10 min followed by 40 cycles of 95℃ for 15 sec and 60℃ for 60 sec in a Light Cycler 480 II (Roche, Switzerland). The threshold cycle (Ct) of each target gene was automatically identified in the linear amplification phase of PCR and normalized to the cycle number of the RNU19 control (ΔCt value). Relative differences in the expression levels of each miRNA sequence were calculated (ΔΔCt) and reported as fold-induction (2-ΔΔCt). The hsa-miRNA primer was: miR-210 5' CUGUGCGUGUGACAGCGGCUGA 3'.
Single-stranded siRNA specific for human HIF-1α (si-HIF-1α) was synthesized by Bioneer (Korea). Sequence of the oligonucleotide was as follows: 5' AGAGGUGGAUAUGUGUGGGdTdT 3'. For transfection, hMSCs were grown to 80% confluence in 10% FBS contained DMEM at 37℃ incubator and then transfected with HIF-1α siRNA using Oligofectin (Invitrogen, USA). Briefly, si-HIF-1α was added to hMSCs at a final concentration of 100 nM in 10% FBS contained DMEM without antibiotics. After transfection for 4 h, the media were replaced in 10% FBS contained DMEM with antibiotics.
hMSCs were lysed in lysis buffer containing phosphate-buffered saline containing 1% Triton X-100, 10× protease inhibitor cocktail (Roche, Switzerland), 1 mM phenylmethylsulfonyl fluoride (Thermo Fisher Scientific, USA), and 60 nM MG132 at 4℃ for 30 min. Protein concentrations were measured using the Bradford Protein Assay Kit (Bio-Rad, USA). The protein samples (60 µg) were then subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The separated proteins were transferred onto polyvinylidene fluoride membranes (EMD Millipore, USA) at 100 mV and 135 mA for 2 h by Power supply (Bio-Rad, USA). The membranes were blocked in Tris-buffered saline-0.1% Tween 20 (TBST) containing 10% nonfat dried milk for 1 h at room temperature or overnight (O/N) at 4℃. Next, the membranes were incubated with 1 : 1,000 anti-HIF-1α, 1:4,000 anti-β-actin, 1:1,000 anti-phospho-Akt, 1:1,000 Akt, 1:1,000 anti-phospho-ERK1/2, and 1:1,000 ERK1/2 antibodies for 1 h at room temperature or O/N at 4℃. The blots were washed in 1× TBST three times at room temperature and subsequently incubated with 1:4,000 horseradish peroxidase-conjugated secondary antibody against mouse or rabbit (Santa Cruz Biotechnology, USA) for 1 h at room temperature. After washing the membranes six times using TBST, immunoreactive proteins were detected using an enhanced chemiluminescence (ECL) system (Amersham Biosciences, UK). ImageJ software (National Institutes of Health, USA) was used for band intensity quantification.
Cell viability was determined with a CCK-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfo phenyl)-2H-tetrazolium, monosodium salt] assay kit (Dojindo, Japan). hMSCs (5 × 103 cells per well) were plated in triplicate wells in 96-well plates (Corning, USA) and incubated for 24 h. The cells were transfected with miR-210 mimic and incubated in a hypoxic chamber for 24 h. The CCK-8 assay kit was diluted with 0.1% FBS contained DMEM and then 100 µL was added to each well and incubated for 2 h at 37℃. The cells were incubated at 37℃ for 2 h. Absorbance was then measured at 450 nm with a spectrophotometer (Bio-Rad, USA).
The activity of HIF-1α and miR-210 expression is increased by hypoxia. We first confirmed that HIF-1α was expressed in hMSCs under hypoxic conditions and with CoCl2 treatment using a Western blot. HIF-1α expression was up-regulated after CoCl2 treatment or with oxygen deprivation (Fig. 1). Until 24 h of hypoxia, HIF-1α expression increased compared to control. To detect miR-210 transcription, we examined changes in miR-210 transcript levels in the presence of CoCl2 or under hypoxic conditions. Changes in miR-210 transcription were the same as those observed for HIF-1α under the same experimental conditions (Fig. 2). Taken together, these data indicate that long-term exposure to hypoxia results in decreased HIF-1α and miR-210 expression.
To investigate the role of miR-210 in regulating HIF-1α expression, hMSCs were transfected with miR-210. Transfection efficiency was determined by real-time PCR (Fig. 3A). As shown in Fig. 3B, miR-210 overexpression induced HIF-1α expression under normoxic conditions and restored the expression of HIF-1α 24 h after hypoxia. These data suggested that an important function of miR-210 is to induce the protein expression of HIF-1α with normoxia and that this function is maintained during hypoxia.
We investigated the link between miR-210 and HIF-1α up-regulation in hypoxic hMSCs. We successfully obliterated HIF-1α expression in hMSCs using siRNA. miR-210 overexpression enhanced HIF-1α protein levels in hypoxic hMSCs (Fig. 4). Furthermore, hypoxic conditions failed to induce miR-210 expression in the hMSCs with a concomitant loss of HIF-1α. Taken together, these results suggested a positive feedback loop between miR-210 and HIF-1α in hypoxic hMSCs.
To further evaluate the effect of miR-210 and HIF-1α on the survival of hypoxic hMSCs, we transfected hMSCs with miR-210 mimics and siRNA specific for HIF-1α. The cells were then maintained under hypoxic conditions. Survival was measured with a CCK-8 assay. After 24 h of incubation with hypoxia, cell death was reduced in the miR-210-transfected cells compared to the other groups (Fig. 5). We also observed greater phosphorylation of Akt and ERK1/2 in the miR-210-transfected hMSCs under hypoxic conditions. Moreover, survival rate was significantly reduced in hMSCs transfected with siRNA specific for HIF-1α (Fig. 6).
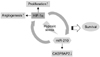
Based on our results, we proposed a model in which HIF-1α induces miR-210 expression that in turn increases the stability of HIF-1α. This positive feedback loop helps to increase HIF-1α levels during low-oxygen conditions and results in increased hMSC survival under hypoxic conditions through enhanced phosphorylation of Akt and ERK1/2 (Fig. 7).
In the present study, we demonstrated the protective effect of miR-210 and HIF-1α in hypoxic hMSCs. Based on our findings, we suggest that a positive feedback loop involving these factors results in increased survival of hMSCs cultured under hypoxic conditions via Akt and ERK signaling. MSCs, which are multipotent stromal cells, have been isolated from various adult tissues such as born marrow, adipose tissue, muscle, and synovial membrane [24]. MSCs are capable of self-renewal and differentiation into multiple cell lineages [3,24,27]. Because of these capacities, MSCs have emerged as the most suitable candidates for use in cell-based therapy and tissue engineering. Despite interest in the clinical applications of MSCs, therapeutic techniques using these cells are limited because of poor MSC viability after cell transplantation. Thus, genetic modification to protect MSCs from apoptosis resulting from hypoxic conditions is necessary to improve the efficacy of MSC-based therapy.
miRNA is endogenous noncoding RNA that down-regulates gene expression by translational inhibition or degradation of target mRNA. The discovery of miRNA resulted in a paradigm shift in the way we view the role of RNA in regulating gene expression [1,7]. miRNA acts in a complex functional network in which each miRNA probably controls hundreds of distinct target genes and the expression of a single coding gene can be regulated by several miRNA molecules [20,22]. miRNA is made in a multi-step process. First, primary miRNA (pri-miRNA) transcripts are produced by RNA polymerase II in the nucleus. pri-miRNA is then transformed into hairpin precursors (pre-miRNA). The pre-miRNA is transported from the nucleus to cytoplasm where it is processed by Dicer to form mature single-stranded miRNA. Finally, a single strand is preferentially loaded into the core binding RNA-induced silencing complex. miRNA binds directly to the 3'-untranslated region of specific mRNA transcripts through complete or partial complementary pairing to induce silencing or direct cleavage of the mRNA. Recent evidence shows the important role of miRNA in regulating a multitude of physiological functions such as stem cell differentiation, neurogenesis, hematopoiesis, immune responses, skeletal and cardiac muscle development, and stress [4,9,11,16,17,26,33,36].
Induction of HIF-1α expression constitutes an integral part of the cellular response to hypoxia. Moreover, HIF-1α serves as a master regulator that orchestrates the expression of genes and helps cells adapt to low oxygen tension. HIF-1α translocates into the nucleus under hypoxic conditions to form a functional complex [30]. With a better understanding of gene regulation by transcription factors with the participation of miRNA, a novel relationship has been established between hypoxia and a select group of miRNA.
The expression of several miRNA transcripts has been found to be regulated by HIF-1α in response to hypoxia Among them, miR-210, a direct transcriptional target of HIF-1α, has emerged as a critical element of the cellular hypoxia response in a broad variety of cell types ranging from cancer cell lines to human umbilical vein endothelial cells [8,12,13]. Interestingly, miR-210 expression is regulated by both HIF-1α [2,5,12,35] and HIF-2α [8]. HIF-1α directly binds to a hypoxia responsive element (HRE) on the proximal miR-210 promoter [12]. When the miR-210 core promoter is compared across species, the HRE is highly conserved, indicating the importance of hypoxia in regulating miR-210 expression [13]. Consistent with this observation, the induction of miR-210 expression with hypoxia is dependent on HIF-1α [5]. miR-210 modulates hypoxia-induced cell cycle arrest [8,12,18]. Recently, miR-210-based gene therapy using mini genes in mice has been shown to improve cardiac function in a model of myocardial infarction [15]. Ischemic preconditioning also augments the survival of stem cells via miR-210 expression by targeting caspase 8-associated protein 2 [8].
Despite these findings, our understanding of the relationship between miR-210 and HIF-1α in hypoxic hMSCs remains limited. The purpose of the present study was to examine mechanisms underlying the induction of miR-210 expression via HIF-1α and evaluate the effect of miR-210 on the survival of hypoxic hMSCs. In conclusion, our results have helped increase our understanding about the relationship between HIF-1α and miR-210. Findings from the current study suggest that the viability of MSCs could be enhanced by increasing miR-210 expression before transplantation. Our data may serve as a basis for developing novel therapeutic modalities for treating injured tissues.
Figures and Tables
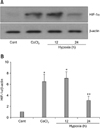 | Fig. 1Expression of hypoxia inducible factor (HIF)-1α in human mesenchymal stem cells (hMSCs) under hypoxic conditions. hMSCs were treated with 250 µM CoCl2 for 24 h and incubated in a hypoxic chamber for 24 h. (A) Expression of HIF-1α was detected by immunoblotting. (B) The ratio of HIF-1α to β-actin band density values. Results are expressed as the mean ± SE for three independent experiments performed in triplicate. *p < 0.01 and **p < 0.05 vs. the control. |
 | Fig. 2Expression of microRNA-210 (miR-210) with hypoxia or CoCl2 treatment. miR-210 expression was measured by real-time PCR. Expression of endogenous miR-210 was detected under hypoxic conditions over time or in the presence with 250 µM CoCl2 for 24 h. Results are expressed as the mean ± SE for three independent experiments performed in triplicate. *p < 0.01 and **p < 0.05 vs. the control. |
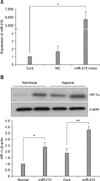 | Fig. 3Effect of miR-210 over-expression on HIF-1α protein stability in hMSCs. (A) Transfection efficiency of the miR-210 mimic was monitored by real-time PCR. (B) Expression of HIF-1α was detected by immunoblotting. Cells transfected with the miR-210 mimic were incubated under normoxic or hypoxic conditions. Results are expressed as the mean ± SE for three independent experiments performed in triplicate. *p < 0.01 and **p < 0.05 vs. the control. |
 | Fig. 4Effect of HIF-1α down-regulation on the induction of miR-210 expression under hypoxic conditions. hMSCs were transfected with HIF-1α-specific small interfering RNA (si-HIF-1α) at a final concentration of 100 nM and incubated in a hypoxia chamber for 12 h. (A) Expression of HIF-1α and β-actin was detected by immunoblotting. (B) miR-210 expression was measured by real-time PCR. Results are expressed as the mean ± SE for three independent experiments performed in triplicate. *p < 0.01 vs. the control. |
 | Fig. 5Effect of miR-210 and HIF-1α on the survival of hypoxic hMSCs. After transfection with the miR-210 mimic, the survival of hMSCs was measured with a CCK-8 assay. Data presented a typical experiment conducted more than three times. Results are expressed as the mean ± SE. *p < 0.01 and **p < 0.05 vs. the
control. |
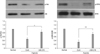 | Fig. 6Effect of miR-210 and HIF-1α on cell survival-related signaling in hypoxic hMSCs. Anti-apoptotic signaling was detected by immunoblotting. Cells were incubated under hypoxic conditions for 24 h. Results are expressed as the mean ± SE for three independent experiments performed in triplicate. *p < 0.01 and **p < 0.05 vs. the control. |
Acknowledgments
This research was supported by a Korea Science and Engineering Foundation Grant funded by the Korea Government (MEST) (2011-0019243, 2011-0019254), a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A120478), and a grant from the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (A085136).
References
1. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004. 116:281–297.
2. Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, Harris AL, Gleadle JM, Ragoussis J. hsa-miR-210 is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res. 2008. 14:1340–1348.

4. Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006. 38:228–233.

5. Crosby ME, Kulshreshtha R, Ivan M, Glazer PM. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res. 2009. 69:1221–1229.

6. De Filippis L, Delia D. Hypoxia in the regulation of neural stem cells. Cell Mol Life Sci. 2011. 68:2831–2844.

7. Dykxhoorn DM. MicroRNAs and metastasis: little RNAs go a long way. Cancer Res. 2010. 70:6401–6406.
8. Fasanaro P, D'Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008. 283:15878–15883.

9. Felli N, Fontana L, Pelosi E, Botta R, Bonci D, Facchiano F, Liuzzi F, Lulli V, Morsilli O, Santoro S, Valtieri M, Calin GA, Liu CG, Sorrentino A, Croce CM, Peschle C. MicroRNAs 221 and 222 inhibit normal erythropoiesis and erythroleukemic cell growth via kit receptor down-modulation. Proc Natl Acad Sci USA. 2005. 102:18081–18086.

10. Geng YJ. Molecular mechanisms for cardiovascular stem cell apoptosis and growth in the hearts with atherosclerotic coronary disease and ischemic heart failure. Ann NY Acad Sci. 2003. 1010:687–697.

11. Hu R, Li H, Liu W, Yang L, Tan YF, Luo XH. Targeting miRNAs in osteoblast differentiation and bone formation. Expert Opin Ther Targets. 2010. 14:1109–1120.

12. Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK, Story M, Le QT, Giaccia AJ. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell. 2009. 35:856–867.

13. Huang X, Le QT, Giaccia AJ. MiR-210--micromanager of the hypoxia pathway. Trends Mol Med. 2010. 16:230–237.
14. Kelly TJ, Souza AL, Clish CB, Puigserver P. A hypoxia-induced positive feedback loop promotes hypoxia-inducible factor 1α stability through miR-210 suppression of glycerol-3-phosphate dehydrogenase 1-like. Mol Cell Biol. 2011. 31:2696–2706.

15. Kim HW, Haider HK, Jiang S, Ashraf M. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem. 2009. 284:33161–33168.

16. Kloosterman WP, Lagendijk AK, Ketting RF, Moulton JD, Plasterk RHA. Targeted inhibition of miRNA maturation with morpholinos reveals a role for miR-375 in pancreatic islet development. PLoS Biol. 2007. 5:e203.

17. Krichevsky AM, Sonntag KC, Isacson O, Kosik KS. Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells. 2006. 24:857–864.

18. Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, Calin GA, Ivan M. A microRNA signature of hypoxia. Mol Cell Biol. 2007. 27:1859–1867.

19. Lei Z, Li B, Yang Z, Fang H, Zhang GM, Feng ZH, Huang B. Regulation of HIF-1α and VEGF by miR-20b tunes tumor cells to adapt to the alteration of oxygen concentration. PLoS One. 2009. 4:e7629.

20. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005. 120:15–20.

21. Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010. 40:294–309.

22. Miranda KC, Huynh T, Tay Y, Ang YS, Tam WL, Thomson AM, Lim B, Rigoutsos I. A pattern-based method for the identification of microRNA binding sites and their corresponding heteroduplexes. Cell. 2006. 126:1203–1217.

23. Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M, Tokudome T, Mori H, Miyatake K, Kitamura S. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005. 112:1128–1135.

24. Orbay H, Tobita M, Mizuno H. Mesenchymal stem cells isolated from adipose and other tissues: basic biological properties and clinical applications. Stem Cells Int. 2012. 2012:461718.

25. Orlic D, Hill JM, Arai AE. Stem cells for myocardial regeneration. Circ Res. 2002. 91:1092–1102.

26. Pedersen IM, Cheng G, Wieland S, Volinia S, Croce CM, Chisari FV, David M. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature. 2007. 449:919–922.

27. Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999. 284:1168–1170.

28. Puisségur MP, Mazure NM, Bertero T, Pradelli L, Grosso S, Robbe-Sermesant K, Maurin T, Lebrigand K, Cardinaud B, Hofman V, Fourre S, Magnone V, Ricci JE, Pouysségur J, Gounon P, Hofman P, Barbry P, Mari B. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ. 2011. 18:465–478.

29. Rane S, He M, Sayed D, Vashistha H, Malhotra A, Sadoshima J, Vatner DE, Vatner SF, Abdellatif M. Downregulation of miR-199a derepresses hypoxia-inducible factor-1α and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009. 104:879–886.

31. Silva GV, Litovsky S, Assad JAR, Sousa ALS, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RVC, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005. 111:150–156.

32. Taguchi A, Yanagisawa K, Tanaka M, Cao K, Matsuyama Y, Goto H, Takahashi T. Identification of hypoxia-inducible factor-1α as a novel target for miR-17-92 microRNA cluster. Cancer Res. 2008. 68:5540–5545.

33. Tay YMS, Tam WL, Ang YS, Gaughwin PM, Yang H, Wang W, Liu R, George J, Ng HH, Perera RJ, Lufkin T, Rigoutsos I, Thomson AM, Lim B. MicroRNA-134 modulates the differentiation of mouse embryonic stem cells, where it causes post-transcriptional attenuation of Nanog and LRH1. Stem Cells. 2008. 26:17–29.

34. Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002. 105:93–98.

35. Zhang Z, Sun H, Dai H, Walsh RM, Imakura M, Schelter J, Burchard J, Dai X, Chang AN, Diaz RL, Marszalek JR, Bartz SR, Carleton M, Cleary MA, Linsley PS, Grandori C. MicroRNA miR-210 modulates cellular response to hypoxia through the MYC antagonist MNT. Cell Cycle. 2009. 8:2756–2768.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download